Anti-atrophic rhinitis and haemophilus parasuis vaccine composition and preparation thereof
The technology of Haemophilus suis and vaccine composition is applied in the field of vaccine composition against swine atrophic rhinitis and Haemophilus parasuis and its preparation, and can solve the problems of inability to protect pigs, asynchronous immunity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
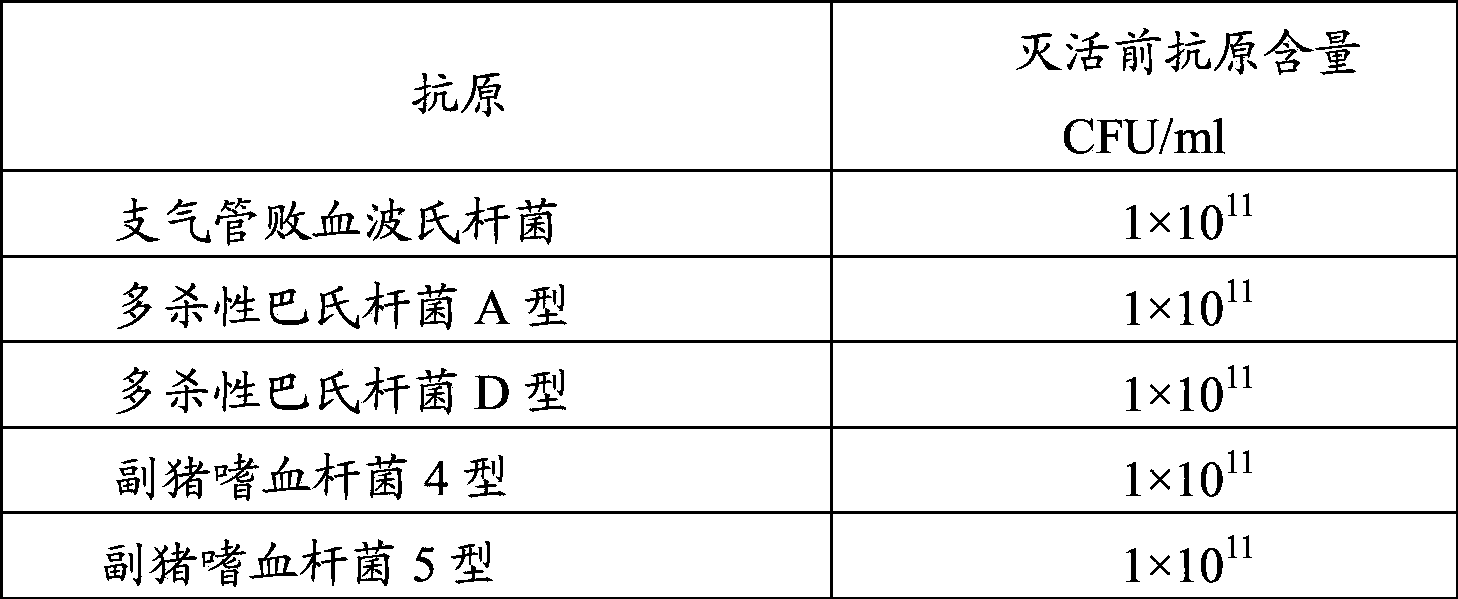
[0061] Example 1: Preparation of porcine bronchiseptica Bordetella antigen, Pasteurella multocida antigen and Haemophilus parasuis antigen
[0062] 1. Source of the strain
[0063] Bordetella bronchiseptica HN8 strain (CCTCC NO: M2011223), Pasteurella multocida type A HN5 strain (CCTCC NO: M2011222) and D type HB4 strain (CCTCC NO: M2011221) used in the manufacture and inspection of this product, Haemophilus parasuis type 4 JS strain (CCTCC M 2011172) and type 5 ZJ strain (CCTCC M 2011173) were both isolated and identified by Pulaike Bioengineering Co., Ltd. Preserved by the Culture Collection Center.
[0064] The strains used in the manufacture and inspection of this product are Bordetella bronchiseptica HN8 strain, Pasteurella multocida type A HN5 strain and D type HB4 strain, Haemophilus parasuis type 4 JS strain and type 5 ZJ strain, All were stored freeze-dried.
[0065] 2. Preparation and inspection of vaccine semi-finished products
[0066] (1) Preparation of seeds ...
Embodiment 2
[0088] Embodiment 2: Preparation of anti-porcine atrophic rhinitis and Haemophilus parasuis disease vaccine composition
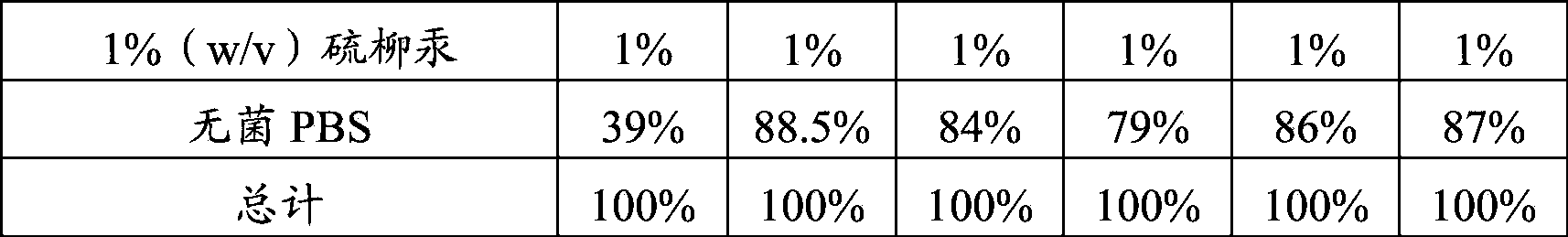
[0089] 1. Preparation of preservatives
[0090] 1% (w / v) thimerosal aqueous solution: 1 g of thimerosal was dissolved in 100 ml of purified water, and autoclaved at 121° C. for 30 minutes for later use.
[0091] 2. Preparation of diluent
[0092] Sterile PBS buffer solution: Dissolve 8g sodium chloride, 0.25g potassium chloride, 3.63g disodium hydrogen phosphate, 0.24g potassium dihydrogen phosphate in 900ml purified water, then dilute to 1L, autoclave at 121°C for 30min spare.
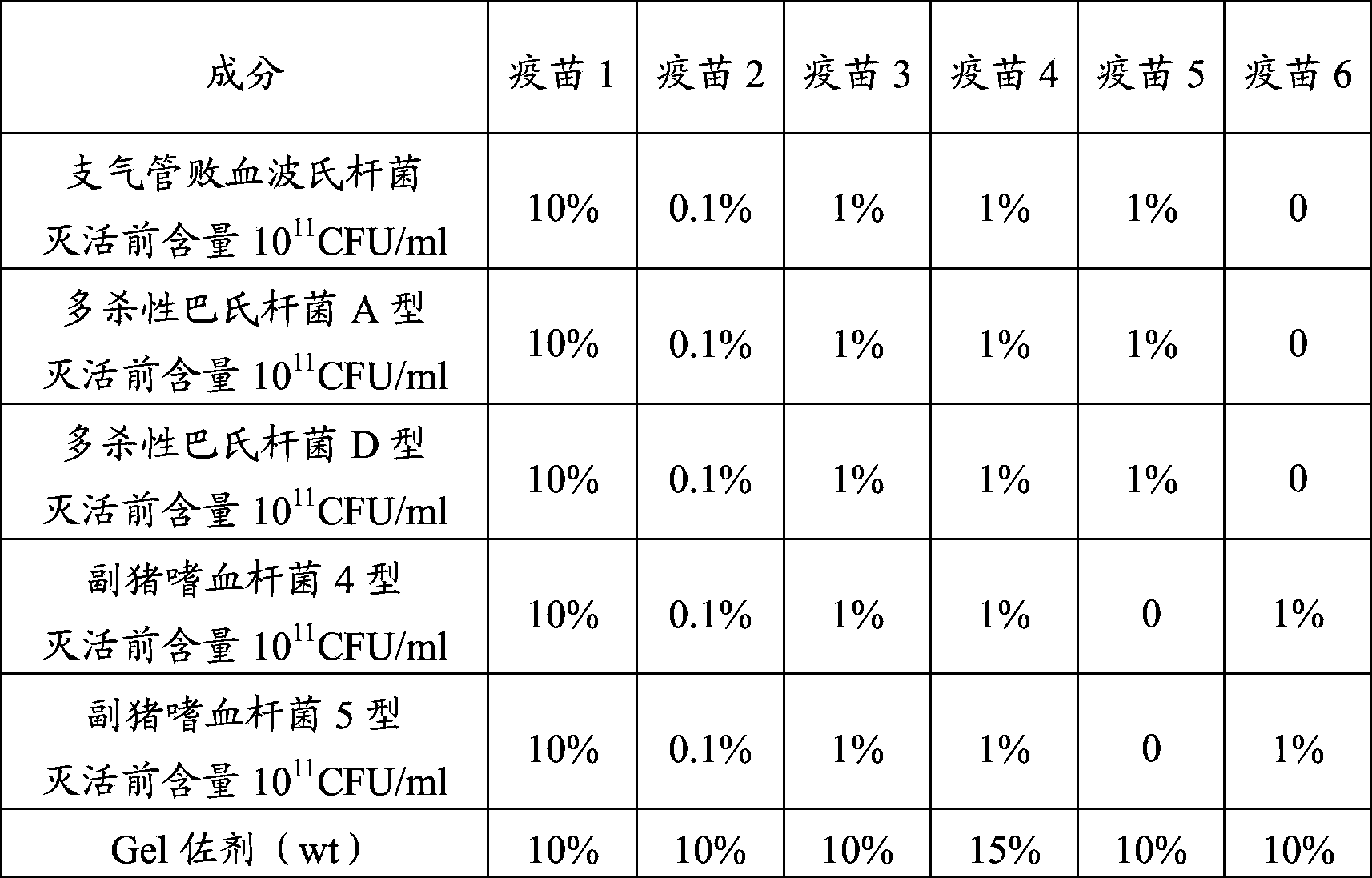
[0093] 3. Vaccine Adjuvant Treatment
[0094] Sterilization of the Gel adjuvant: transfer the Gel adjuvant into a sterilizable container, and autoclave at 121°C for 30 minutes for later use.
[0095] 4. Matching seedlings
[0096] Mix the above ingredients in a certain proportion, that is, through aseptic operation, the concentrated antigen of Bordetella bronchiseptica, the co...
Embodiment 3
[0100] Example 3: Anti-porcine atrophic rhinitis and Haemophilus parasuis vaccine composition efficacy test with different antigen contents
[0101] 1. Test material
[0102] According to embodiment 2 laboratory preparation anti-porcine atrophic rhinitis and Haemophilus parasuis vaccine composition, vaccine 1 (bordetella porcine bronchiseptica antigen content 10 10 CFU / ml, Pasteurella multocida type A antigen content 10 10 CFU / ml, Pasteurella multocida type D antigen content 10 10 CFU / ml, Haemophilus parasuis type 4 antigen content 10 10 CFU / ml, Haemophilus parasuis type 5 antigen content 10 10 CFU / ml) and vaccine 2 (Bordetella porcine bronchiseptica antigen content 10 8 CFU / ml, Pasteurella multocida type A antigen content 10 8 CFU / ml, Pasteurella multocida type D antigen content 10 8 CFU / ml, Haemophilus parasuis type 4 antigen content 10 8 CFU / ml, Haemophilus parasuis type 5 antigen content 10 8 CFU / ml).
[0103] Weaned piglets aged 3 to 4 weeks without antibodies to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com