Antigen composition for preventing and treating pig secondary infected respiratory diseases and its preparation method and application
A technology for respiratory diseases and secondary infection, applied in the field of veterinary vaccines, can solve the problems of decreased efficacy of live vaccines, high stress in pigs, and high viscosity of oily adjuvants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
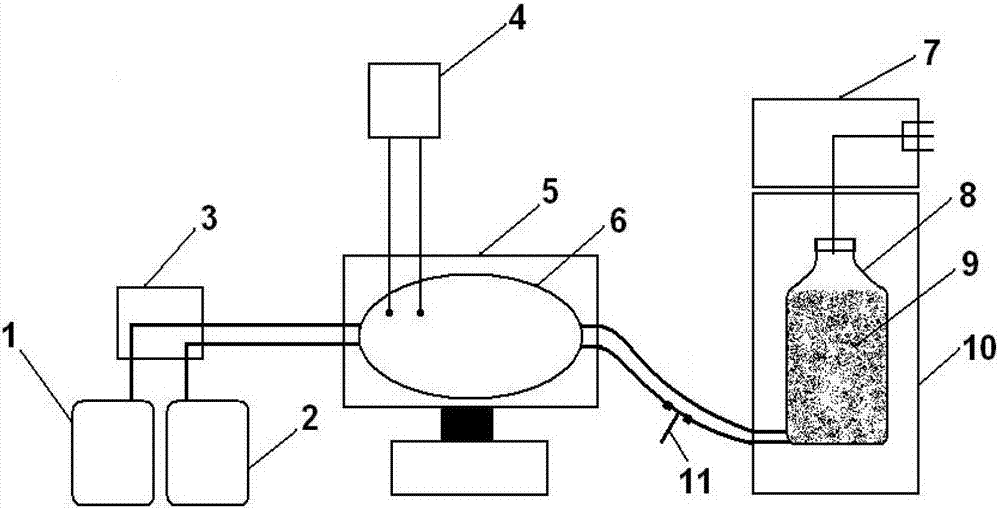
[0069] The present invention will be further described below in conjunction with specific embodiments, and the advantages and characteristics of the present invention will become clearer along with the description. However, these embodiments are only exemplary and do not constitute any limitation to the scope of the present invention. Those skilled in the art should understand that the details and forms of the technical solutions of the present invention can be modified or replaced without departing from the spirit and scope of the present invention, but these modifications and replacements all fall within the protection scope of the present invention. Example 1 Tidal microcarrier suspension Large-scale Production of Porcine Reproductive and Respiratory Syndrome Virus and Preparation of Vaccine in Buoyant Bioreactor
[0070] The microcarrier used in the present embodiment is polyester fiber, and the virus strain that is used to prepare reproductive and respiratory syndrome ...
Embodiment 2
[0085] The screening of embodiment 2 adjuvants
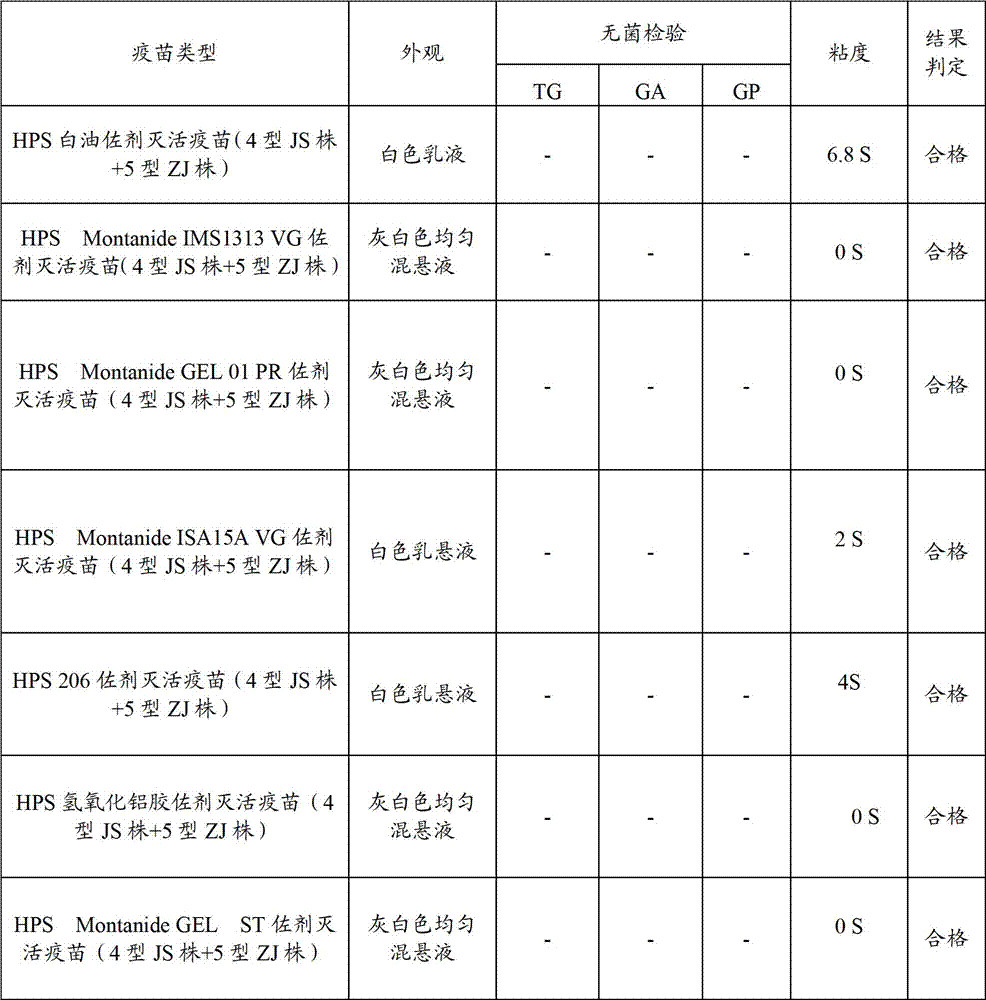
[0086] Using Haemophilus parasuis serum type 4 JS strain, type 5 ZJ strain antigen, respectively with white oil adjuvant, 206 adjuvant, nano aluminum hydroxide gel adjuvant, Montanide IMS1313 VG, MontanideISA15A VG, Montanide GEL 01 PR and Montanide GEL ST polymer adjuvant was formulated separately, and the antigen content of the finished vaccine was 2.0×10 for the serum type 4 JS strain and type 5 ZJ strain before inactivation. 9 CFU / head serving. The prepared vaccine was tested for compatibility with the PRRS vaccine prepared in Example 1 after the character test, the safety test and the potency test.
[0087] (1) Trait test (see Table 2)
[0088] Table 2 Appearance, sterility test and viscosity determination of vaccines with various adjuvants
[0089]
[0090] (2) Safety: The prepared vaccine was injected intramuscularly into 5 healthy susceptible pigs, 4mL each. Within 14 days, some vaccine groups had local or system...
Embodiment 3
[0104] Embodiment 3 Haemophilus parasuis inactivated vaccine (JS strain+ZJ strain) preparation
[0105] Haemophilus parasuis inactivated vaccine (JS strain + ZJ strain), which has been inactivated, ultra-filtered and passed the security inspection, uses Haemophilus parasuis serum type 4 JS strain, type 5 ZJ strain antigen, and MontanideGEL 01 ST The adjuvant is mixed and prepared, wherein the ratio of the above two antigens is 1:1, accounting for 90% of the total volume of the vaccine, the content of the adjuvant is 10%, and the antigen content of the finished vaccine is 4 JS strains and 5 ZJ strains before inactivation. The number of live bacteria was 2.1×10 9 CFU / head serving.
[0106] 1. Strains
[0107] 1.1 The JS strain of Haemophilus parasuis serotype 4 was isolated and identified by Pulaike Bioengineering Co., Ltd., and has been preserved in the China Center for Type Culture Collection. The preservation date is May 18, 2011, and the preservation number is: CCTCC M2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com