Gel polymer electrolyte and preparation method thereof
A technology of gel polymer and electrolyte, which is applied in the direction of hybrid capacitor electrolyte, hybrid/electric double layer capacitor manufacturing, etc., which can solve the problems of reduced stability of electric double layer capacitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
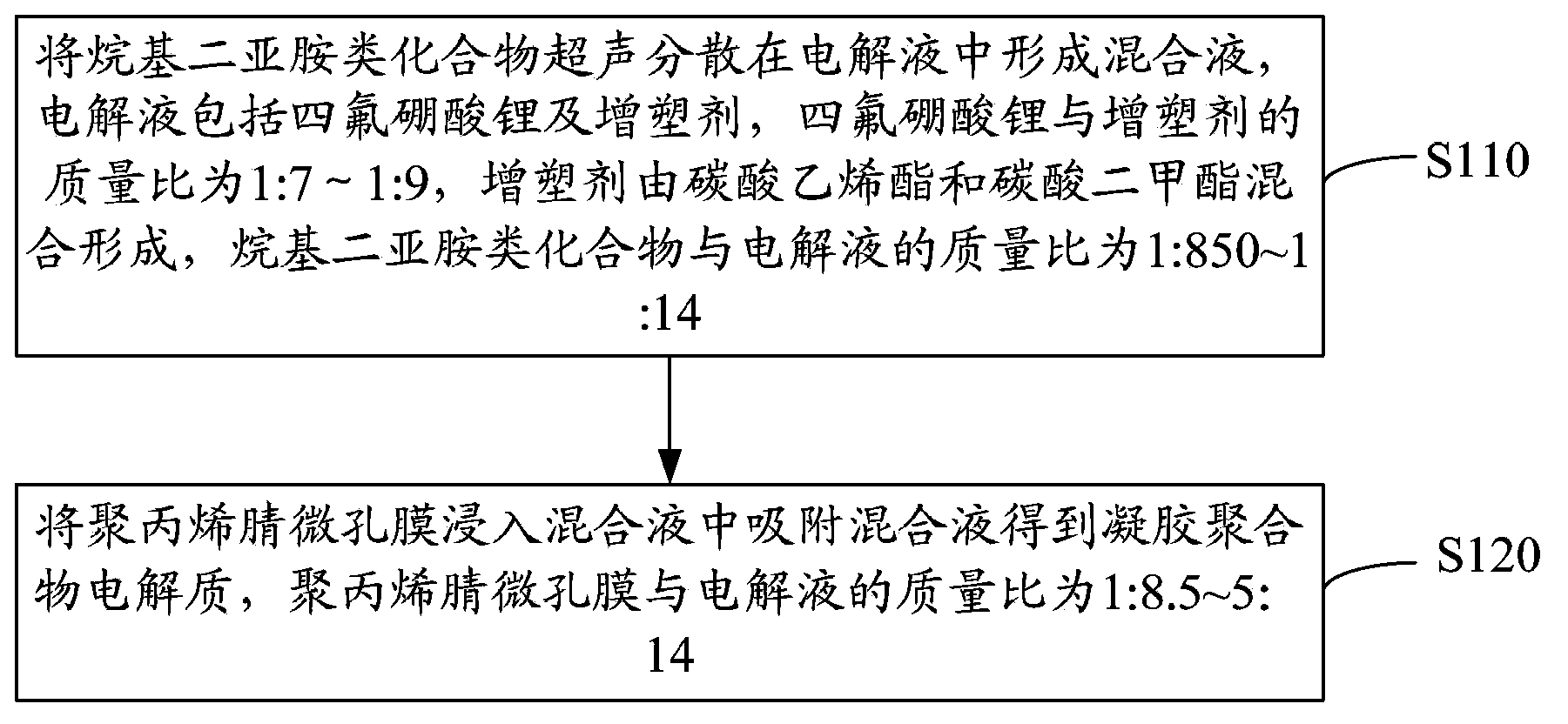
[0025] see figure 1 , the preparation method of above-mentioned gel polymer electrolyte, comprises the following steps:
[0026] Step S110, ultrasonically dispersing the alkyldiimine compound in the electrolytic solution to form a mixed solution, the electrolytic solution includes lithium tetrafluoroborate and a plasticizer, and the mass ratio of lithium tetrafluoroborate to the plasticizer is 1:7-1 :9, the plasticizer is formed by mixing ethylene carbonate and dimethyl carbonate, and the mass ratio of alkyldiimine compound to electrolyte is 1:850~1:14.
[0027] Preferably, the alkyldiimine compound is selected from one of N,N'-dicyclohexylcarbodiimide and N,N'-dicyclopentylcarbodiimide.
[0028] Preferably, the volume ratio of ethylene carbonate to dimethyl carbonate is 1:1˜1:3.
[0029] Preferably, the time for ultrasonic dispersion is 5 minutes to 30 minutes.
[0030] Step S120, immersing the polyacrylonitrile microporous membrane in the mixed solution to absorb the mixe...
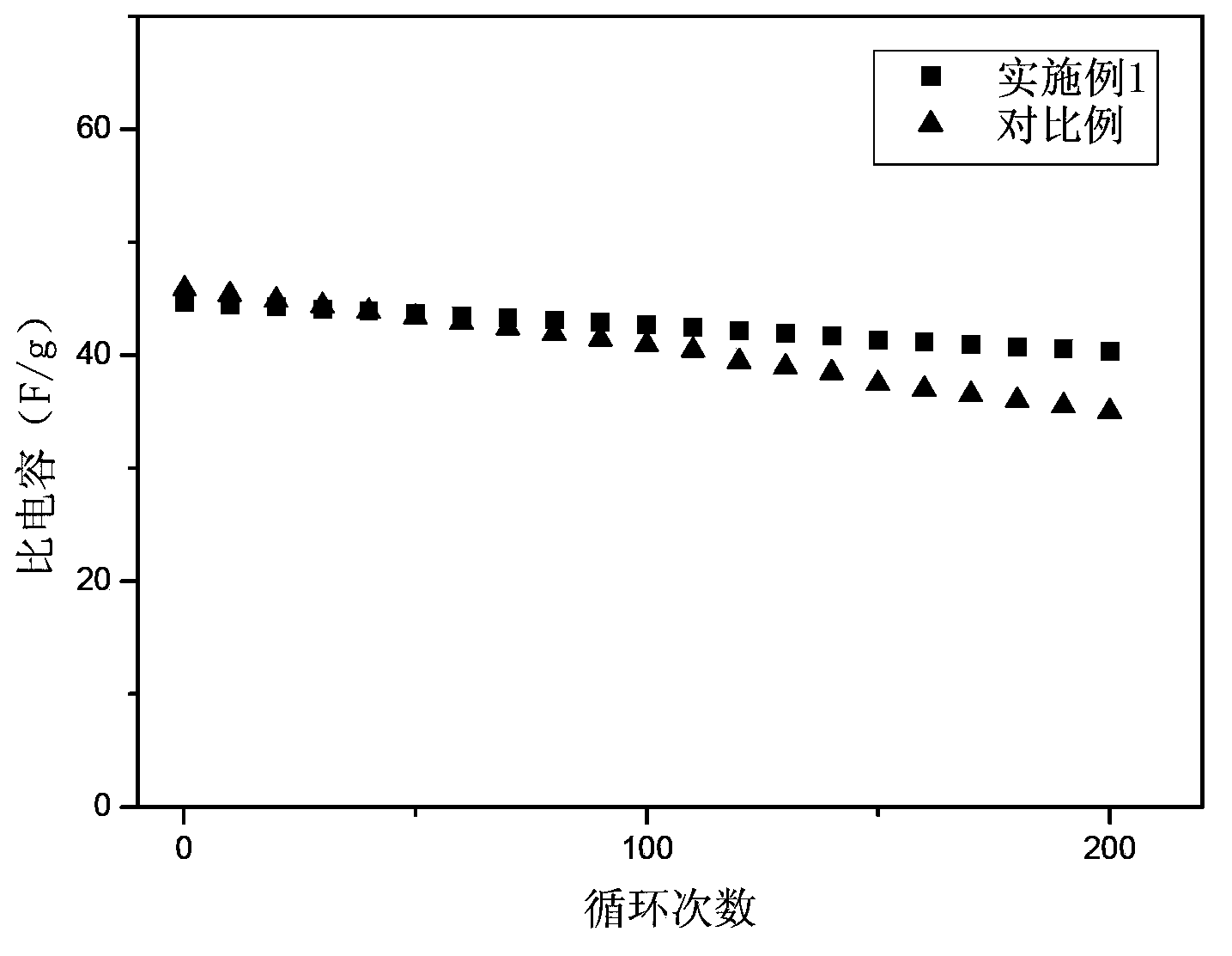
Embodiment 1
[0036] In the glove box, ultrasonically disperse 0.5g N,N'-dicyclohexylcarbodiimide (ultrasonic 30min) in 8.5g lithium salt solution to form a mixed solution, wherein the lithium salt solution includes LiBF4 and plasticizer, LiBF4 and plasticizer The mass ratio of the plasticizer is 1:8, and the plasticizer is formed by mixing EC and DMC with a volume ratio of 1:2, and then immersing 1g of the dried PAN microporous membrane in the above mixed solution, and after 180min, the PAN microporous membrane will The mixed solution was completely absorbed to obtain the PAN-based gel polymer electrolyte added with N,N'-dicyclohexylcarbodiimide. In the obtained gel polymer electrolyte, the mass percentage of PAN matrix is 10%, the mass percentage of lithium salt and plasticizer is 85%, and the mass percentage of alkyldiimine compound is 5%.
Embodiment 2
[0038] In the glove box, ultrasonically disperse 0.4g N,N'-dicyclopentylcarbodiimide (ultrasonic 25min) in 8.4g lithium salt solution to form a mixed solution, wherein the lithium salt solution includes LiBF4 and plasticizer, LiBF4 and The mass ratio of the plasticizer is 1:9, and the plasticizer is formed by mixing EC and DMC at a volume ratio of 1:1, and then immersing 1.2g of the dried PAN microporous membrane in the above mixture, and taking it out after 150min , that is, a PAN-based gel polymer electrolyte added with N,N'-dicyclopentylcarbodiimide was obtained. In the obtained gel polymer electrolyte, the mass percentage of PAN matrix is 12%, the mass percentage of lithium salt and plasticizer is 84%, and the mass percentage of alkyldiimine compound is 4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com