Anticancer genetic engineering bivalent antibody, preparation method thereof, and anticancer genetic engineering drug
A genetic engineering and anti-tumor technology, applied in the field of biopharmaceuticals, can solve the problems of limited specific binding between anti-tumor antibodies and tumor cells, and ineffective tumor prevention and treatment, and achieve prevention and treatment of tumor cell infection and specific binding Enhanced, crafted simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
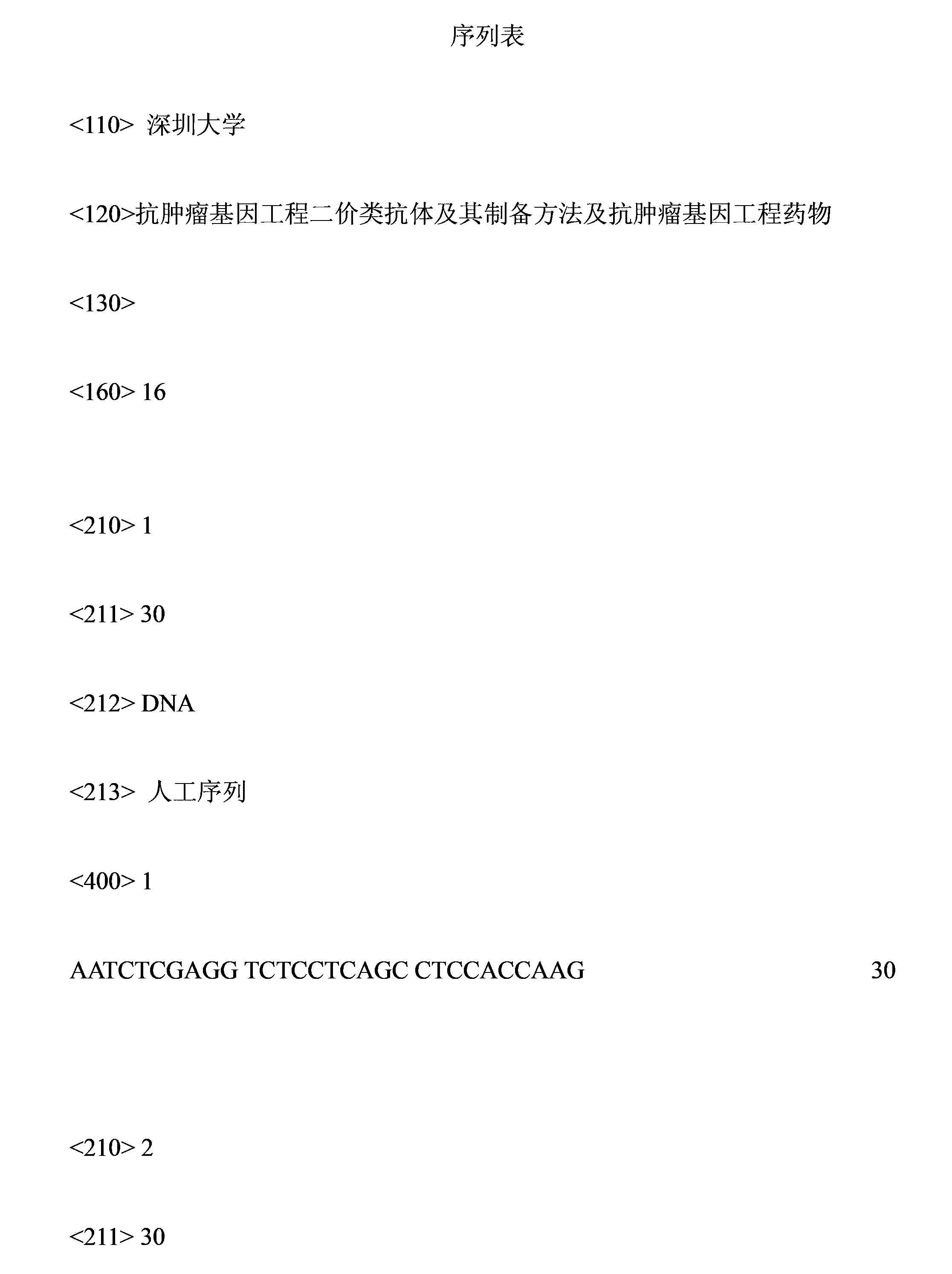
[0024] The preparation method of the anti-tumor genetic engineering bivalent antibody of the present invention comprises the following steps: A: obtaining the constant region gene, the SIRPα gene and the B7 gene respectively; B: when the constant region gene is the constant region gene of a human antibody, using The eukaryotic expression vector constructs an expression plasmid comprising the constant region gene of the human antibody, the SIRPα gene and the B7 gene; or, when the constant region gene is a flexible linker sequence, a prokaryotic expression vector or the eukaryotic expression vector is used to construct an expression plasmid comprising a flexible linker sequence. The expression plasmids of the connecting sequence, SIRPα gene and B7 gene; C. Transfect the expression plasmid formed in step B into the expression cell line for culturing, and stably express the bivalent antibody; D. Collect the supernatant by centrifugation or purify the cells Obtain bivalent antibodie...
Embodiment 1
[0051] The PCR product of the constant region gene of the IgG1 antibody was digested by Xho I and Xba I, and then loaded into the eukaryotic expression vector pCDNA3.1 / His C, which was digested with the same restriction enzymes, to construct a recombinant plasmid pCDNA3 containing the constant region gene of the IgG1 antibody .1 / His C-IgG; clone the SIRPα gene and B7 gene amplified by PCR into the recombinant plasmid pCDNA3.1 / His C-IgG containing the constant region gene of IgG1 antibody, and construct the constant region gene containing IgG1 antibody Eukaryotic expression plasmids pCDNA3.1 / His C-SIRPα-IgG and pCDNA3.1 / His C-B7-IgG flexibly linked to SIRPα gene and B7 gene. Specifically, the above-mentioned flexible connection is realized through a flexible connection sequence (GAA TGT TCC).
[0052] Using 293 cells as the expression cell line, high-quality and high-concentration eukaryotic expression plasmids pCDNA3.1 / His C-SIRPα-IgG and pCDNA3.1 / His C-B7-IgG were extracted, ...
Embodiment 2
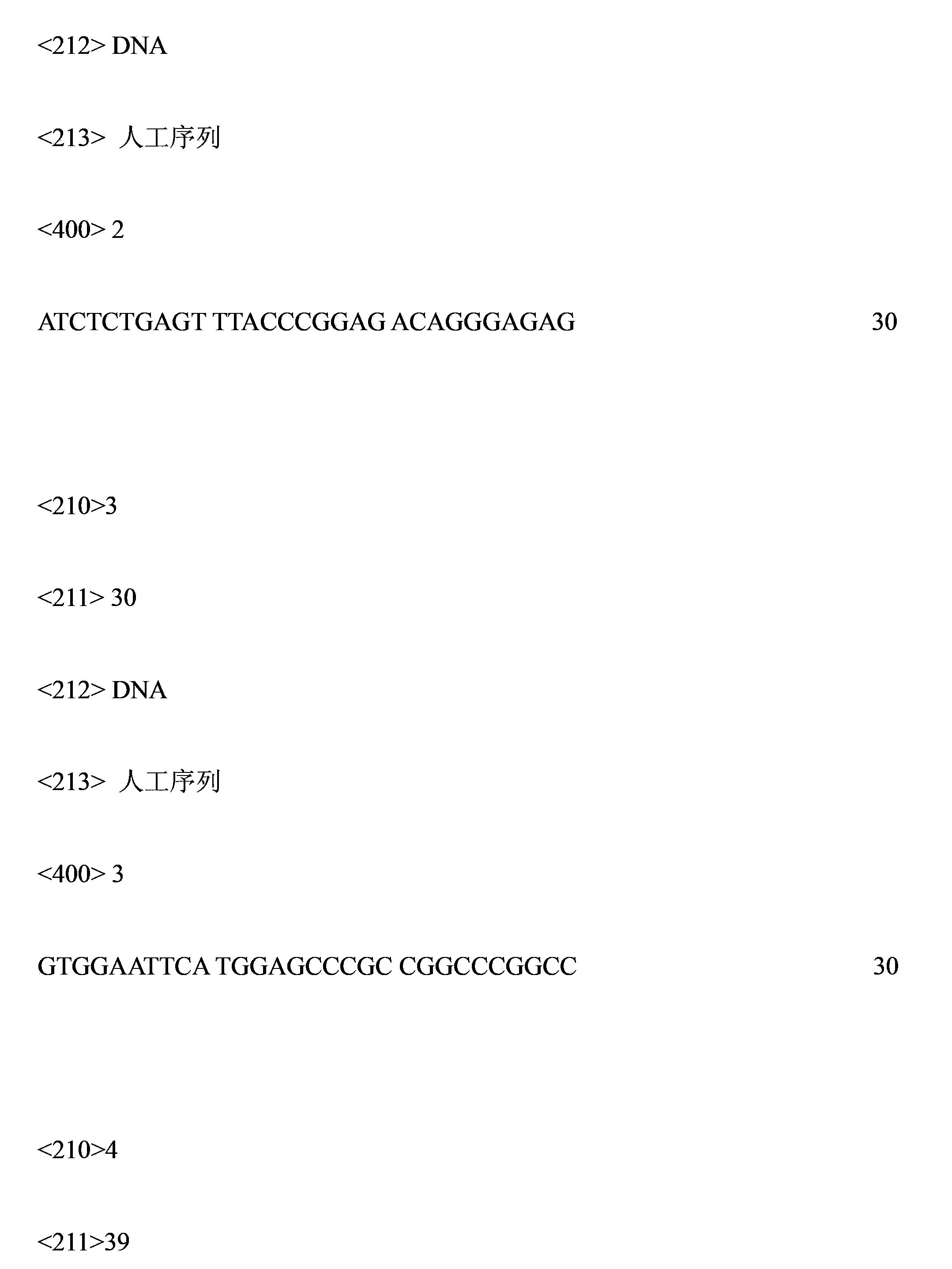
[0054] Design primers, primer sequence 9 is: 5'-CTT AAG CTT ACC ATG GGG GGT TCT-3', from the eukaryotic expression plasmid pCDNA3.1 / His C-SIRPα-IgG and pCDNA3.1 / His C- The SIRPα-IgG and B7-IgG genes of the eukaryotic expression vector pCDNA3.1 / His C with selection tags (6×His tag and Xpress Epitop tag) were amplified from B7-IgG, and these two genes were loaded into pCDNA5 / In the eukaryotic vector of FRT / TO TOPO TA, the eukaryotic expression plasmids pCDNA5 / FRT / TO TOPO TA-SIRPα-IgG and pCDNA5 / FRT / TO TOPO TA-B7-IgG were obtained.
[0055] The human kidney epithelial cell line Flp-In T-Rex 293 was used as the expression cell line to establish a stable and high-expression cell line, and extract high-quality and high-concentration eukaryotic expression plasmids pCDNA5 / FRT / TO TOPO TA-SIRPα-IgG and pCDNA5 / FRT / TO TOPO TA-B7-IgG was transfected into Flp-In T-Rex 293 cells using liposome transfection method. After 24 hours, the antibiotic Blasticidin B was used to screen for resistan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com