A kind of synthesis technology of vildagliptin
A synthetic method and technology of pyrrolidine, applied in the direction of organic chemistry, can solve the problems of unfavorable industrialization, many by-products, poor crystallization and separation effect, etc., and achieve great economic and social benefits, simple equipment, and green production procedures. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
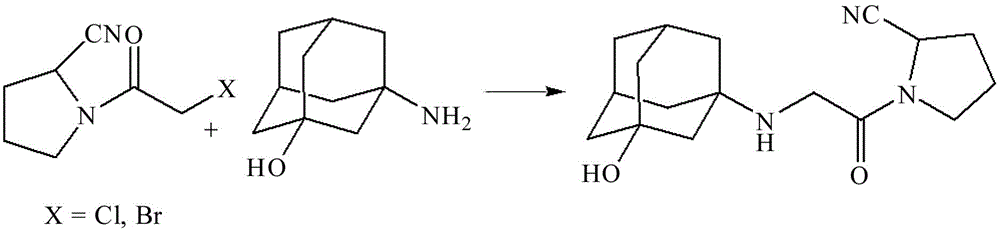
[0021] Synthesis of embodiment 1 vildagliptin 1
[0022]In No. 1 500L stainless steel reaction kettle, add (S)-1-(2-chloroacetyl)pyrrolidine-2-cyanide 20.0kg and 3-hydroxy-adamantanamine 22.0kg, add dichloromethane 160L and stir dissolve. Add 10.0 kg of neutral alkali potassium carbonate and 1.1 kg of tetrabutylammonium bromide into No. 2 stainless steel reaction kettle, and then stir and dissolve with 80.0 kg of water. The jacket is passed cold brine to control the temperature of the No. 1 reactor below 10°C, and the aqueous solution of the No. 2 reactor is slowly introduced, and the addition is completed in about 2 hours. Then the temperature was slowly raised to room temperature, and the stirring reaction was continued for 10 h until the raw material (S)-1-(2-chloroacetyl)pyrrolidine-2-cyanide was completely reacted as monitored by thin-layer chromatography, and the reaction was terminated. The reaction solution was transferred to an extraction tank to stand for stratific...
Embodiment 2
[0023] Synthesis 2 of embodiment 2 vildagliptin
[0024] In No. 1 500L stainless steel reaction kettle, add (S)-1-(2-bromoacetyl)pyrrolidine-2-cyanide 21.5kg and 3-hydroxy-adamantanamine 22.0kg, add dichloromethane 160L and stir dissolve. Add 9.0kg of neutral alkali sodium carbonate and 1.2kg of tetrabutylammonium bromide into No. 2 stainless steel reaction kettle, and then stir and dissolve with 80.0kg of water. The jacket is passed cold brine to control the temperature of the No. 1 reactor below 10°C, and the aqueous solution of the No. 2 reactor is slowly introduced, and the addition is completed in about 2 hours. Then slowly raise the temperature to 20-22°C, and continue to stir the reaction for 5 hours until the raw material (S)-1-(2-bromoacetyl)pyrrolidine-2-cyanide is completely reacted as monitored by thin layer chromatography, and the reaction is terminated. The reaction solution was transferred to an extraction tank to stand for stratification, the dichloromethane ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com