Preparation method of surface covered nickel lithium manganate positive electrode material
A technology of lithium nickel manganate and cathode material, which is applied in the field of preparation of lithium nickel manganate cathode material to achieve the effect of shortening microwave firing time, improving cycle performance and rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A preparation method of a surface-coated lithium nickel manganese oxide positive electrode material, comprising the following steps:
[0032] a. Configure the bottom liquid of the reaction kettle, add a solvent to the nickel source compound and the manganese source compound to dissolve, according to the stoichiometric ratio LiNi of lithium nickel manganese oxide 0.5 mn 1.5 o 4 Configure soluble nickel salt and manganese salt solutions respectively, and add 30% nickel salt solution or manganese salt solution into the reactor as the bottom liquid of the reactor;
[0033] b. Prepare co-precipitate, configure a precipitant solution with a concentration of 0.5-2.5mol / L, add the precipitant solution and the above-mentioned remaining nickel salt solution or manganese salt solution into the bottom liquid of the reaction kettle in a drop-by-drop manner and stir to generate a co-precipitant solution. Precipitation reaction to obtain a co-precipitation mixture, the addition rate...
Embodiment 1
[0042] Take by weighing 1314g nickel sulfate, 2265g manganese sulfate respectively, after adding 20L deionized water to dissolve completely, drip concentration is 2mol / L (NH 4 ) 2 CO 3 Solution until the precipitation is complete, the co-precipitation reaction temperature is maintained at 50°C, the stirring rate is 1000rpm, and the co-precipitation reaction is carried out for 3 hours.
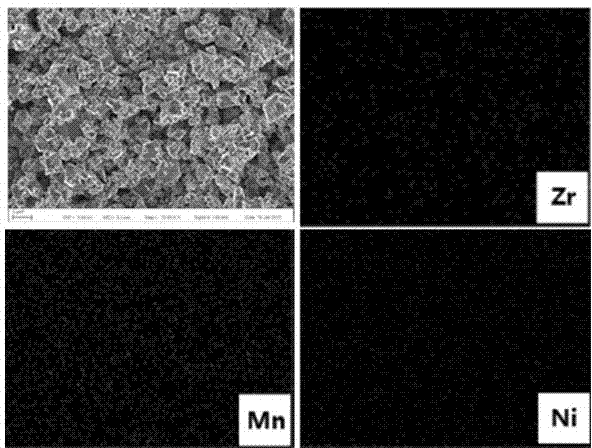
[0043] The precipitate was filtered and washed three times with water, and then transferred to a blast drying oven at 100° C. for drying for 6 hours. Take zirconia according to the mass ratio: nickel-manganese coprecipitate (3:97), and weigh Li according to the stoichiometric ratio 2 CO 3 , mixed and ball milled for 3 hours.
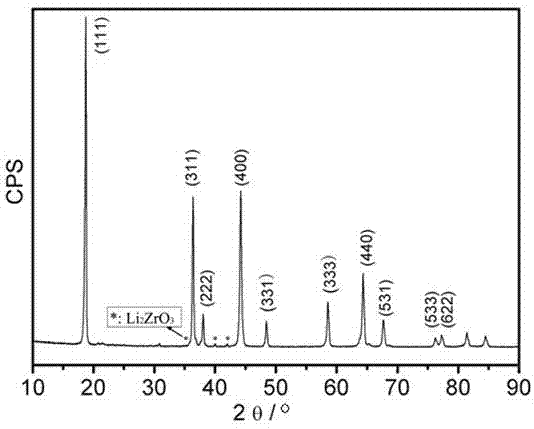
[0044] The resulting precursor was made into a disc under a pressure of 2 MPa with a thickness of 2 cm. Place the sample piece in a microwave high-temperature reaction furnace to react, and within 8 minutes, the material block absorbs microwaves and heats up to 800°C...
Embodiment 2
[0047] Weigh 442g of nickel acetate and 1298g of manganese acetate respectively, add 10L of deionized water to dissolve completely, and drop Na at a concentration of 1.5mol / L within 0.5 hours. 2 CO 3 Solution until the precipitation is complete, the co-precipitation reaction temperature is maintained at 50°C, the stirring rate is 600rpm, and the co-precipitation reaction is carried out for 3 hours.
[0048] The precipitate was filtered and washed three times with water, and then transferred to a blast drying oven at 100° C. for drying for 6 hours. Take zirconia according to the mass ratio: nickel-manganese coprecipitate (2:98), and weigh Li according to the stoichiometric ratio 2 CO 3 , mixed and ball milled for 3 hours.
[0049] The obtained precursor was made into a disc with a thickness of 3 cm under a pressure of 2 MPa. Place the sample piece in a microwave high-temperature reaction furnace to react, and within 8 minutes, the material block absorbs microwaves and hea...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com