Cobalt catalyst for preparing alcohol by highly selective hydrogenation of carboxylic acid
A hydrogenation catalyst and catalyst technology, applied in the chemical industry, can solve the problems of harsh operating conditions, low space-time yield, high reaction pressure, etc., and achieve the effects of low cost, good stability, and environmentally friendly composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Get 1 liter of cobalt nitrate aqueous solution of 1mol / L, add the precipitated silica 40g (silicon dioxide (SiO 2 ) content%≥95.0, fineness (325 mesh sieve residue)%≤1.8, specific surface area is 400~600m 2 / g), the temperature was raised to 60 degrees, and 20wt% sodium carbonate aqueous solution was added under the condition of vigorous stirring until the pH value was 9.0. Gradually raise the temperature to 85° C., keep the constant temperature for 2 hours, add 3.0 g of lanthanum nitrate, continue to keep the temperature for 2 hours, cool down to room temperature, and slowly add 6.0 g of boric acid. After washing and filtering, and drying overnight to obtain a dry filter cake, 10 ml of silver nitrate solution containing 0.1 wt % was sprayed into the filter cake and then granulated. The particles were calcined and decomposed, and then pelletized to obtain the catalyst CHZ-91.
Embodiment 2
[0060] Take 1 liter of 1.2mol / L cobalt nitrate aqueous solution, add 1.0 g of silver nitrate and 10.2 g of copper nitrate to it, after fully dissolving, add concentrated ammonia water (containing NH 3 Mass percentage is about 28%) 0.6L, forming a transparent complex solution. Raise the temperature to 70°C, keep the temperature constant for 2 hours, then slowly add it into 500g of diluted water glass aqueous solution (with a silicon dioxide content of about 10wt%), and then add dropwise 100ml of calcium nitrate solution (calcium nitrate concentration 1.0 mol / L), and continue to stir at constant temperature for 12 hours, add 8 g of boric acid and gradually cool the slurry to room temperature. The above slurry was filtered, washed, and dried overnight to obtain a dry filter cake. The filter cake was roasted and decomposed, and then pelletized to obtain the catalyst CHZ-92.
Embodiment 3
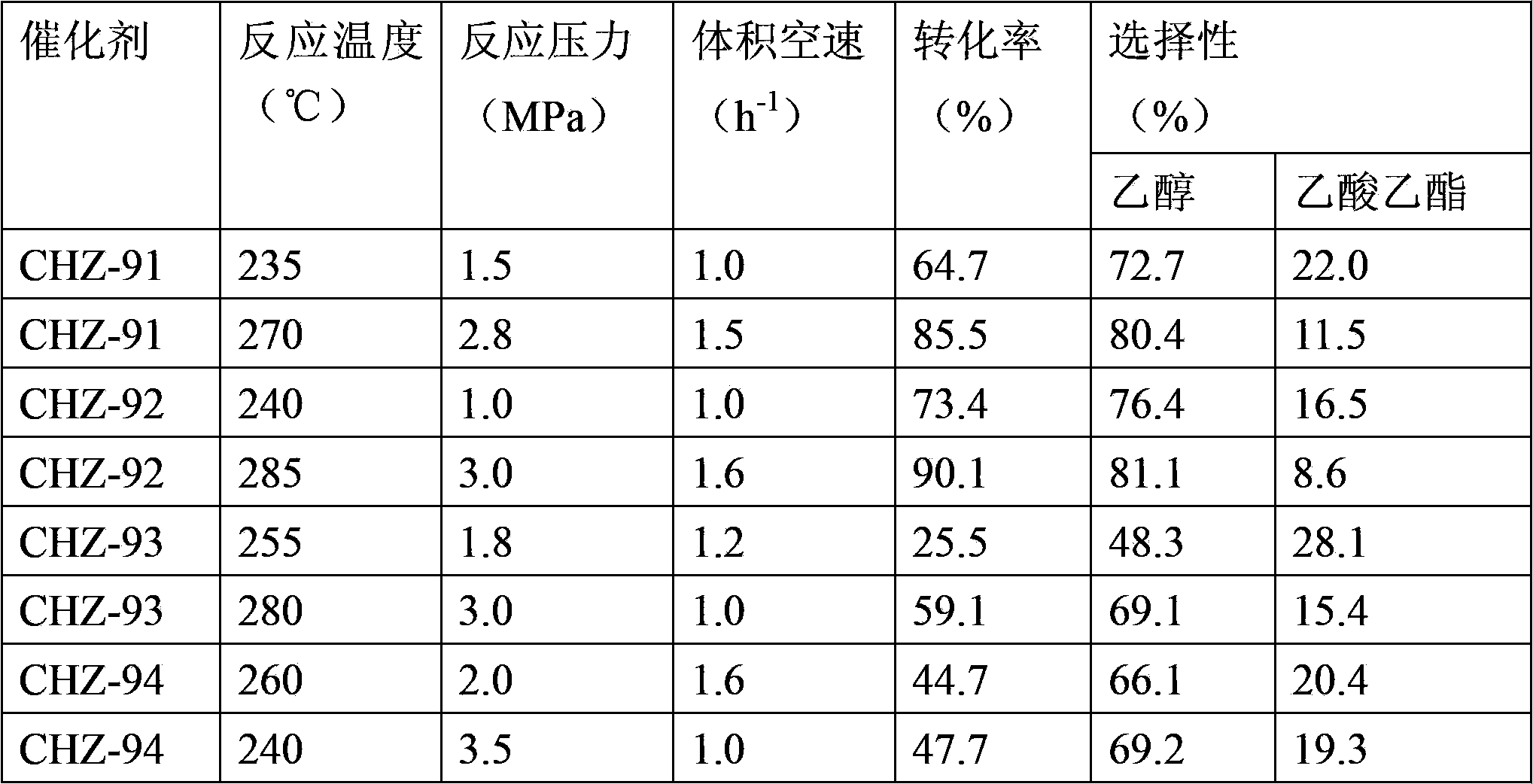
[0066] The above catalyst was evaluated in a fixed-bed reactor for the hydrogenation of acetic acid to produce ethanol. The filling volume was 10ml, diluted with 1:1 quartz sand, and reduced with pure hydrogen before use. The maximum reduction temperature was 450~500°C , the specific reaction conditions and reaction results are shown in Table 1.
[0067] In this example, the acetic acid conversion rate and ethanol selectivity were calculated according to the carbon mole percentage of each component.
[0068]
[0069]
[0070] Other products are: acetaldehyde, ethane, methane, carbon monoxide, carbon dioxide, acetic aldehyde, acetone, propanol, etc.;
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com