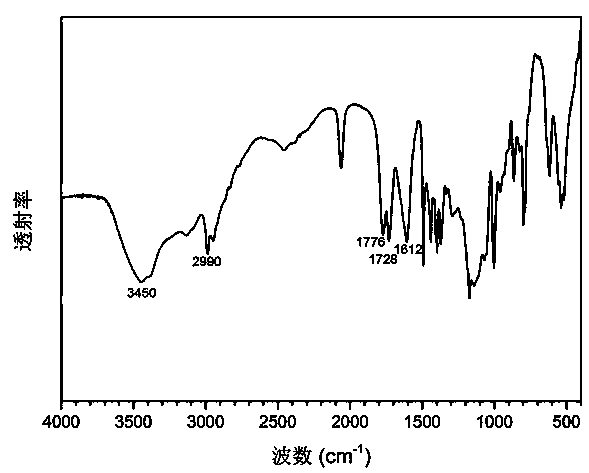
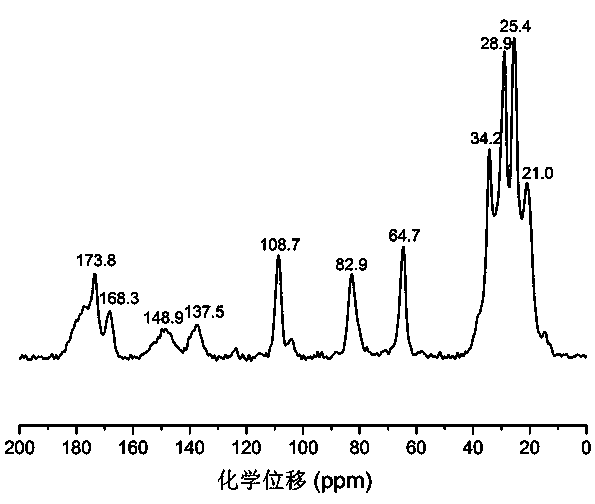
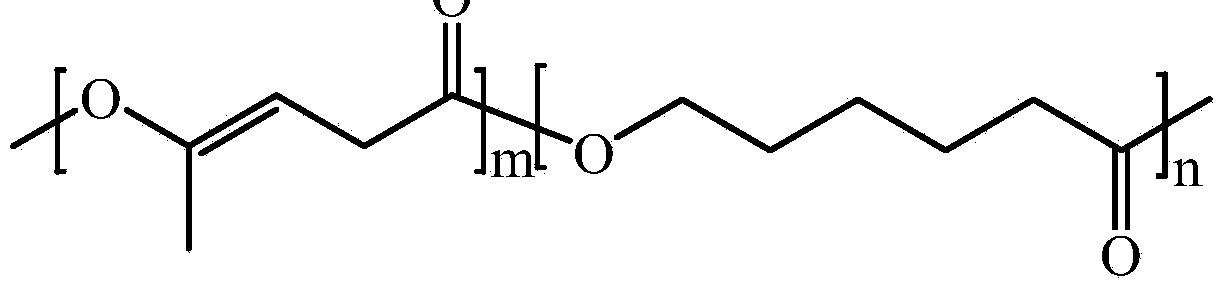Poly(alpha-angelica lactone-co-epsilon-caprolactone) and preparation method thereof
A technology of angelica lactone and caprolactone, applied in the field of polyester polymer synthesis, to achieve a wide range of applications, expand the application range, and improve thermal stability and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Under the protection of high-purity nitrogen, 5.88g (60mmol) α-angelica lactone, 6.84g (60mmol) ε-caprolactone and 0.04g (0.1mmol) stannous octoate were added to the reaction system in sequence. carry out copolymerization. Heated in an oil bath to increase the reaction temperature to 100° C., and reacted for 50 hours. After the reaction was completed, a crude poly(α-angelicalide-co-ε-caprolactone) product was obtained. Using methanol washing and centrifugal separation, the unreacted monomers and oligomers in the crude product of poly(α-angelicalide-co-ε-caprolactone) were removed to obtain poly(α-angelicalide-co -ε-caprolactone) products, the number average molecular weight range of the polyester is 3000-100000.
Embodiment 2
[0027] Under the protection of helium, 9.80g (100mmol) α-angelica lactone, 11.4g (100mmol) ε-caprolactone and 0.04g (0.1mmol) tetraphenyltin were added to the reaction system in sequence. carry out copolymerization. Heated in an oil bath to increase the reaction temperature to 150° C., and reacted for 30 hours. After the reaction was completed, a crude product of poly(α-angelicalide-co-ε-caprolactone) was obtained. Using acetonitrile washing and centrifugal separation, the unreacted monomers and oligomers in the crude product of poly(α-angelicalide-co-ε-caprolactone) were removed to obtain poly(α-angelicalide-co -ε-caprolactone) products, the number average molecular weight of the polyester ranges from 10,000 to 800,000.
Embodiment 3
[0029] Under the protection of argon, 9.80g (100mmol) α-angelica lactone, 11.4g (100mmol) ε-caprolactone and 0.4g (1mmol) triethylaluminum were added to the reaction system in sequence and carried out in a solvent-free system. copolymerization. Heated in an oil bath to increase the reaction temperature to 180° C., and reacted for 60 hours. After the reaction was completed, a crude product of poly(α-angelicalide-co-ε-caprolactone) was obtained. Washing with acetone and centrifuging to remove unreacted monomers and oligomers in the crude product of poly(α-angelicalide-co-ε-caprolactone) to obtain poly(α-angelicalide-co -ε-caprolactone) products, the number average molecular weight of the polyester ranges from 20,000 to 1,000,000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com