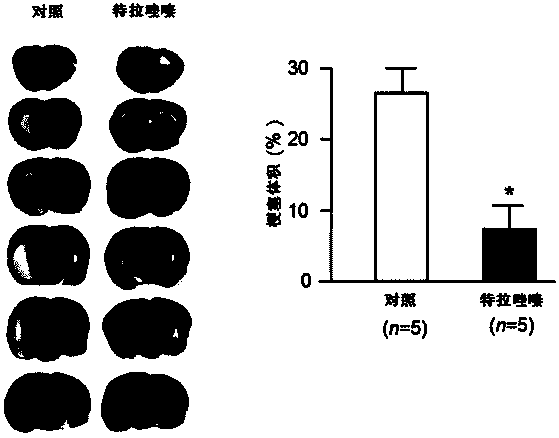
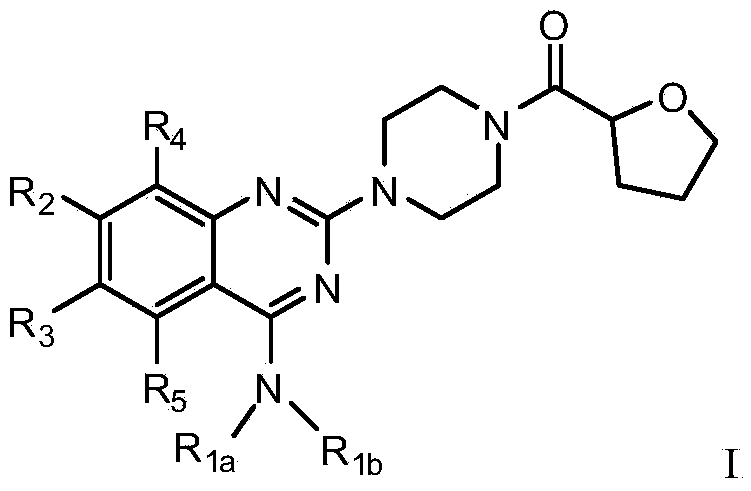
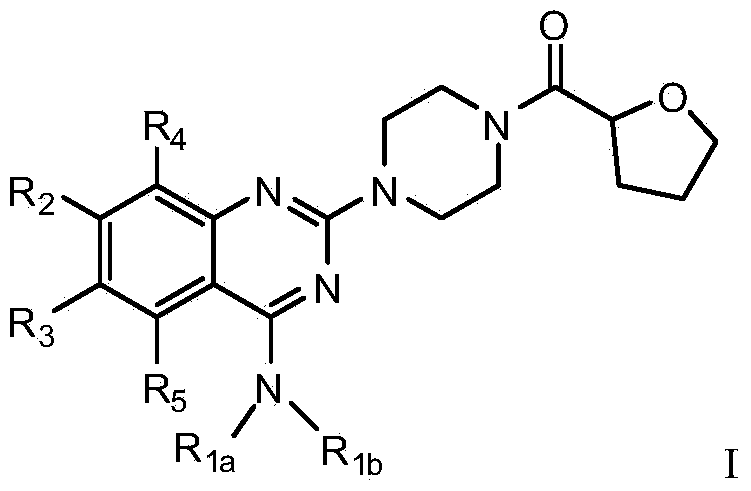
Quinazoline derivative used for cardio cerebrovascular diseases
A technology of compounds and hydrates, applied in the field of quinazoline derivatives such as terazosin, preparation of drugs for cardiovascular and cerebrovascular diseases, and new pharmaceutical applications of quinazoline derivatives, which can solve heavy economic burdens and patients and family issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0081] Preparation Example 1: Preparation of Co.1
[0082]
[0083] Step 1: Bubble ammonia gas into a solution of compound 1a (50 mmol) in 200 mL THF, and react at 25°C for 36 hours. A large amount of white solids were precipitated in the system, and the resulting white solids were filtered and washed with tetrahydrofuran to obtain the final product 1f. Yield: 63%.
[0084]
[0085] Step 2: 15 mL of acetic anhydride was added to compound 1f (10 mmol), and the reaction was refluxed for 2 hours. After cooling to room temperature, a large amount of white solids were precipitated in the system. The resulting white solids were filtered and washed with tetrahydrofuran to obtain 1 g of the final product. Yield: 63%.
[0086]
[0087] Step 3: To a solution of 1 g (2 mmol) of 1-pentanol was added under argon atmosphere for 1 h (2 mmol). After the reaction system was refluxed for 4.5 hours, it was placed in an environment of 0-5° C. for static crystallization. The obtained...
preparation example 2
[0088] Preparation Example 2: Preparation of Co.2
[0089]
[0090] Step 1: Compound 1b (20 mmol) was added to compound 1a (20 mmol) in 100 mL of methanol solution, and the reaction was carried out at 25° C. for 4 hours. Thin plate chromatography indicated that 1a was completely converted, and 100 mL of diethyl ether was added to the system, mixed evenly, and placed in a -20°C environment for static crystallization. The resulting white solid was recrystallized from petroleum ether / ethyl acetate to obtain the final product 1c. Yield: 41%.
[0091]
[0092] Step 2: To a solution of 1c (2 mmol) in 1-pentanol was added 1d (2 mmol) under argon atmosphere. After the reaction system was refluxed for 4.5 hours, it was placed in an environment of 0-5° C. for static crystallization. The obtained white crystals were washed twice with 10 mL of acetone, and then recrystallized with ether / methanol to obtain the final product 1e, which was compound Co.2. Yield: 62%. 1H NMR (300MHz...
preparation example 3
[0093] Preparation Example 3: Preparation of Co.3
[0094]
[0095] Step 1: Add hydrazine hydrate (20 mmol) to a solution of compound 1a (20 mmol) in 100 mL of methanol, and react at 25° C. for 4 hours. Thin plate chromatography indicated that 1a was completely converted, and 100 mL of diethyl ether was added to the system, mixed evenly, and placed in a -20°C environment for static crystallization. The obtained white solid was recrystallized from petroleum ether / ethyl acetate to obtain the final product 3a. Yield: 61%.
[0096]
[0097] Step 2: To a solution of 3a (2 mmol) in 1-pentanol was added 1d (2 mmol) under argon atmosphere. After the reaction system was refluxed for 4.5 hours, it was placed in an environment of 0-5° C. for static crystallization. The obtained white crystals were washed twice with 10 mL of acetone, and then recrystallized with ether / methanol to obtain the final product 3b, which is compound Co.3. Yield: 31%. 1H NMR(300MHz,DMSO-d6):d1.87(m,2H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com