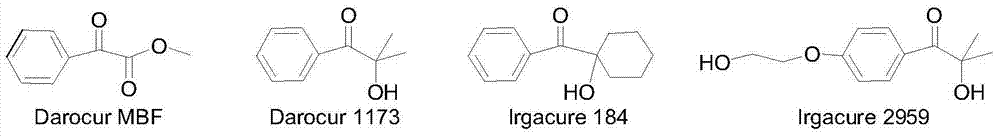
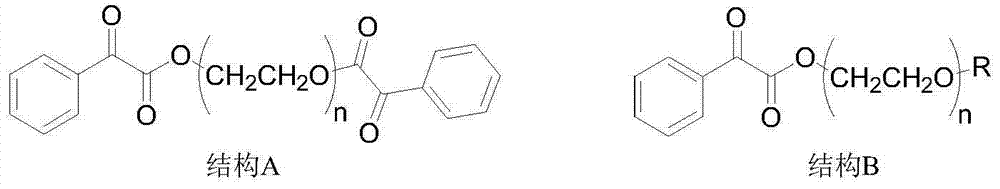
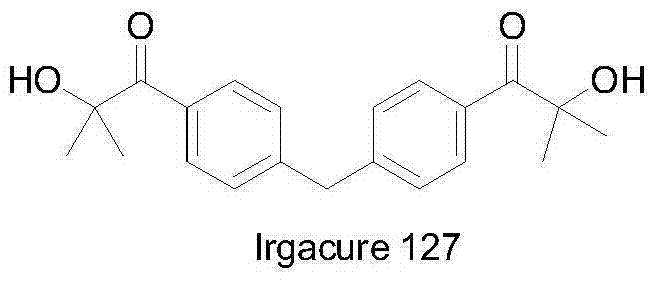
Novel bifunctional benzoyl formic acid hydroxy ketone ester compounds and photoinitiators containing the compounds
A kind of technology of hydroxy ketone group benzoylformate, compound, applied in the field of photoinitiator, can solve the problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of 4-(2-hydroxy-2-methylpropionyl)phenylbenzoylformate (Compound 1)
[0046]
[0047] 2-Hydroxy-2-methyl-1-(4-hydroxyphenyl)-1-propanone (22.5g, 0.125mol) was dissolved in 50ml of dichloromethane, triethylamine (19.0g, 0.188mol) was added, Under the condition of stirring, heat to 60 ℃, add dropwise methyl benzoylformate (20.5g, 0.125mol), keep warm after dropping, monitor the reaction with thin layer chromatography or liquid phase, after the reaction is complete, cool down to room temperature, use Wash with an equal volume of water, dry, and desolventize. The obtained residue is dissolved in dichloromethane, and purified on a silica gel column with petroleum ether / ethyl acetate as eluent to obtain a light yellow product.
[0048] Elemental analysis: Molecular formula C 18 h 16 o 5
[0049] Theoretical content: carbon element content 69.22%; hydrogen element content 5.16%
[0050] Measured content: carbon element content 69.11%; hydrogen elem...
Embodiment 2
[0051] Example 2: Preparation of 4-(2-hydroxy-2-methylpropionyl)phenylbenzoylformate (Compound 1)
[0052]
[0053] 1) Preparation of benzoyl chloride
[0054] Add benzoylformic acid (30.0g, 0.2mol) and 50ml of thionyl chloride into the reaction vessel, stir and reflux the reaction, recover the excess thionyl chloride after 2 hours, and the remaining raffinate can be extracted by vacuum distillation or The next reaction was carried out directly without purification.
[0055] 2) Preparation of phenyl benzoylformate
[0056] Dissolve the unpurified benzoyl chloride prepared above in 120ml of dichloromethane, add phenol (20.6g, 0.22mol) and 30ml of triethylamine and stir the reaction at room temperature. Phase monitoring reaction, after the reaction is complete, add an equal volume of water to wash, separate the organic phase, dry with anhydrous sodium sulfate, remove the solvent, distill under reduced pressure (3mmHg), collect the fraction at 145 ° C to obtain 40.7g of benz...
Embodiment 3
[0064] Example 3: Preparation of 2-(4-(2-hydroxy-2-methylpropionyl)phenoxy)ethylbenzoylformate (Compound 2)
[0065]
[0066] Dissolve methyl benzoylformate (16.4g, 0.1mol) in 50ml of toluene, add pyridine (15.8g, 0.2mol), heat to 70°C under stirring conditions, add Irgacure2959 (22.4g, 0.1mol), After dripping and keeping warm for about 20 hours, monitor the reaction with thin-layer chromatography or liquid phase. After the reaction is complete, cool down to room temperature, wash with an equal volume of water, dry, and remove the solvent. After the residue is dissolved in dichloromethane, use it on a silica gel column. Petroleum ether / ethyl acetate was used as the eluent to obtain a light yellow product.
[0067] Elemental analysis: Molecular formula C 20 h 20 o 6
[0068] Theoretical content: carbon element content 67.41%; hydrogen element content 5.66%
[0069] Measured content: carbon element content 67.23%; hydrogen element content 5.60%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com