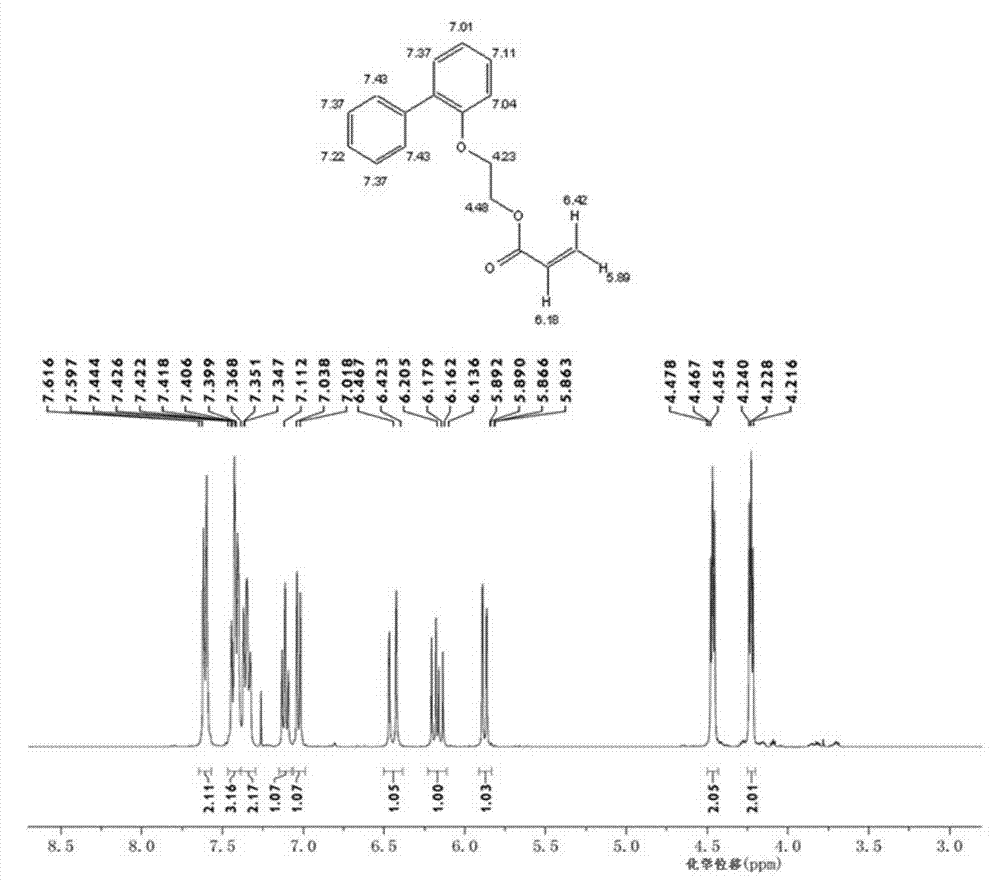
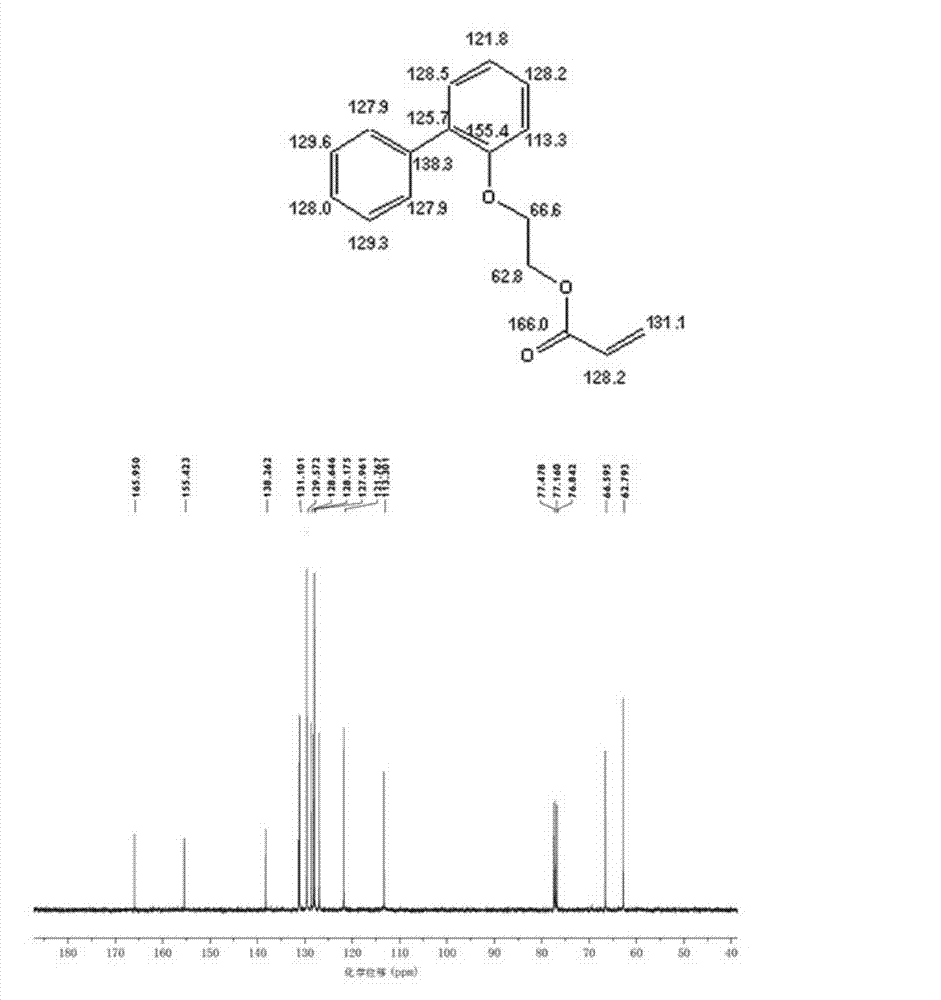
O-phenyl phenoxyethyl acrylate preparation method
A technology of o-phenylphenoxyethyl acrylate and phenylphenoxyethyl acrylate, which is applied in the field of acrylate preparation, can solve the problems of limited effect and increased production cost, and achieve cost saving, low chroma, The effect of low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 128.60 grams of 2-(2-biphenyl)ethanol, 56.23 grams of acrylic acid, 193 grams of cyclohexane, and 2.89 grams of methanesulfonic acid in a 500 ml four-necked flask equipped with a mechanical stirrer, a condenser and a water separator. 0.48 grams of p-hydroxyanisole, 0.37 grams of 50 wt % hypophosphorous acid aqueous solution, nitrogen protection has been passed through during the reaction, and the reaction was refluxed at 80-85 ° C for 6 hours. Generate about 10.8 milliliters of water, continue to reflux for 2 hours, and the water volume remains unchanged.
[0033] After the reaction, the reaction mixture was washed twice with 5% aqueous sodium hydroxide solution, then washed three times with distilled water, and finally dried with anhydrous magnesium sulfate, and then 0.2 p-hydroxyanisole, a polymerization inhibitor, was added to the dried solution. gram, at 50 DEG C, vacuum degree is under 0.1MPa condition, removes solvent cyclohexane with rotary evaporator, obtain...
Embodiment 2
[0035] Add 128.60 grams of 2-(2-biphenyl)ethanol, 56.23 grams of acrylic acid, 193 grams of cyclohexane, and 2.89 grams of methanesulfonic acid in a 500 ml four-necked flask equipped with a mechanical stirrer, a condenser and a water separator. 0.48 grams of p-hydroxyanisole, 3.7 grams of 50 wt% hypophosphorous acid aqueous solution, nitrogen protection has been passed through during the reaction, and the reaction was refluxed at 80-85 ° C for 6 hours. Generate about 10.8 milliliters of water, continue to reflux for 2 hours, and the water volume remains unchanged.
[0036] After the reaction finished, the reaction mixture was washed 2 times with 5% aqueous sodium hydroxide solution, then washed 3 times with distilled water, and finally dried with anhydrous magnesium sulfate, and then 0.2 grams of polymerization inhibitor p-hydroxyanisole was added in the solution after drying , at 50 DEG C, vacuum is 0.1MPa condition, remove solvent cyclohexane with rotary evaporator, obtain c...
Embodiment 3
[0038] Add 128.60 grams of 2-(2-biphenyl)ethanol, 56.23 grams of acrylic acid, 193 grams of cyclohexane, and 2.89 grams of methanesulfonic acid in a 500 ml four-necked flask equipped with a mechanical stirrer, a condenser and a water separator. 0.48 grams of p-hydroxyanisole, 7.4 grams of 50 wt% hypophosphorous acid aqueous solution, nitrogen protection has been passed through during the reaction, and the reaction was refluxed at 80-85 ° C for 6 hours. Generate about 10.8 milliliters of water, continue to reflux for 2 hours, and the water volume remains unchanged.
[0039]After the reaction finished, the reaction mixture was washed 2 times with 5% aqueous sodium hydroxide solution, then washed 3 times with distilled water, and finally dried with anhydrous magnesium sulfate, and then 0.2 grams of polymerization inhibitor p-hydroxyanisole was added in the solution after drying , at 50 DEG C, vacuum is 0.1MPa condition, remove solvent cyclohexane with rotary evaporator, obtain co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com