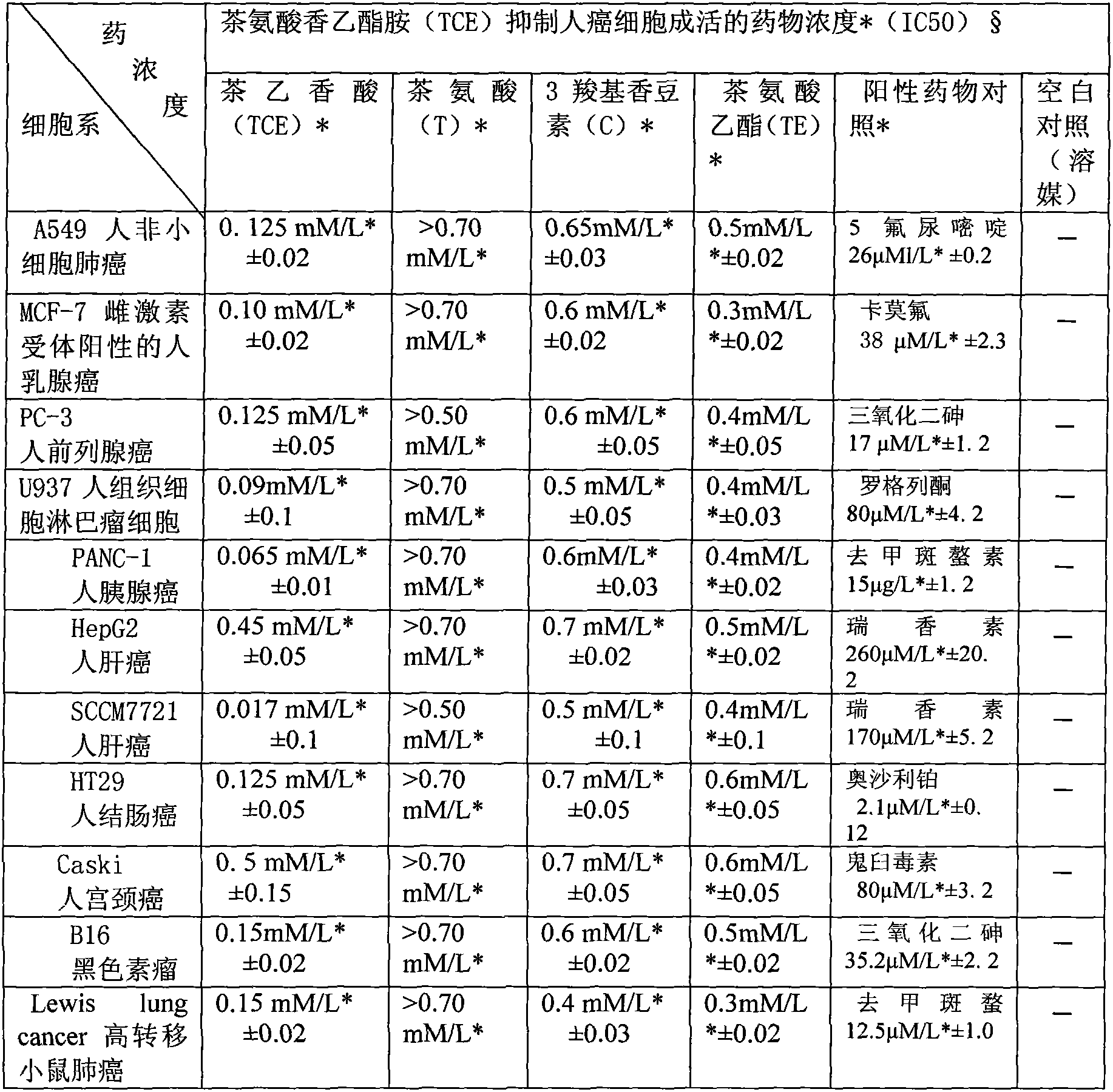
Application of ethyl coumarin-3-carboxylyl L-theanine and the like in preparation of product used for preventing and treating disease such as cancers
A technology of tea ethyl aromatic acid and ethyl theanine, applied in the medical field, can solve the problems of limited prevention and treatment, toxic and side effects, etc., and achieve the effect of enhancing curative effect, less toxic and side effects, and wide indications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
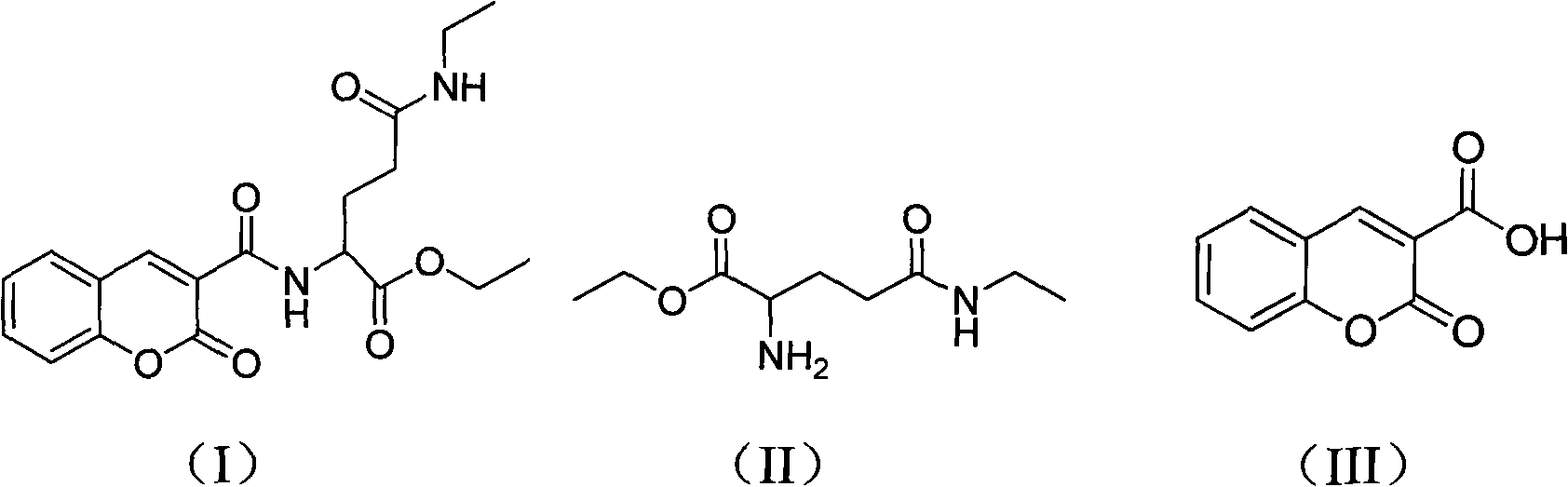
[0035] A compound for treating tumors in this embodiment, its name is ethyl 5-ethylamino-5-oxo-2-(2-oxo-2H-benzopyran-3-carboxamido)pentanoate, Abbreviated as tea ethyl aromatic acid (TEC), it has the chemical structural formula shown in formula (I):
[0036] As the compound shown in formula (I), the physical properties are white powdery solid, and the melting point decomposes above 180 ° C;
[0037]The compound shown in formula (I) is a pharmaceutically acceptable carrier; the pharmaceutically acceptable carrier includes any substance suitable for injection, preferably water for injection, or emulsifier, liposome, nano preparation and other pharmaceutical carriers.
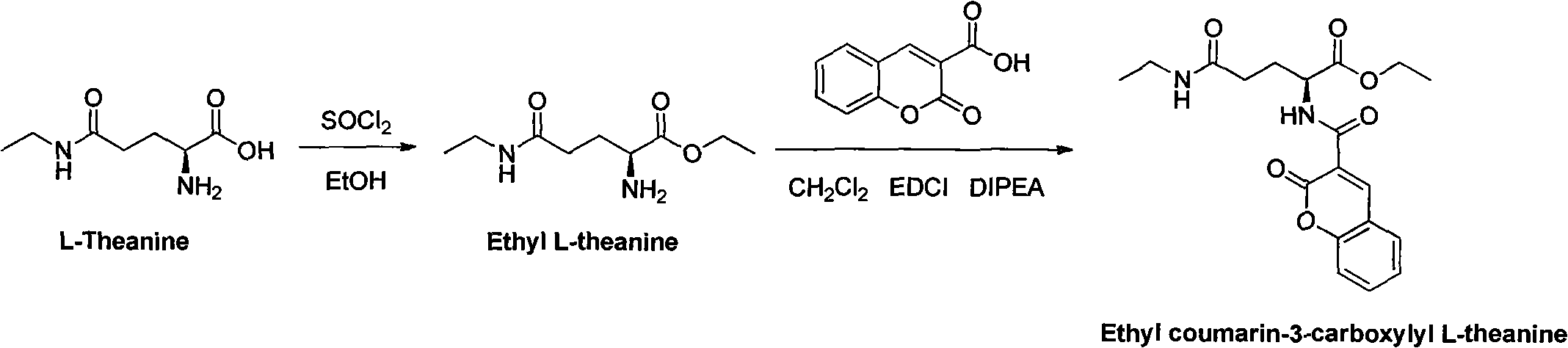
[0038] The preparation method of compound thea ethyl aromatic acid as shown in formula (I), mainly comprises the following steps:
[0039] 1, the preparation of intermediate ethyl theanine, as shown in structural formula (II);
[0040] 2. Further synthesis of thea ethyl aromatic acid by 3-carboxylic acid couma...
Embodiment 2
[0048] The difference between this embodiment and embodiment 1 is that
[0049] Step 1: Preparation of ethyl theanine
[0050] Theanine is dissolved in ethanol at a ratio of 88g / L (referring to dissolving 88g of theanine per liter of ethanol), and then slowly adding thionyl chloride to the system with a volume ratio of 56ml (referring to the dissolving solution of theanine and ethanol and two volume ratio of thionyl chloride), the mixture was stirred at room temperature for 1.5 hours, and the resulting mixture was concentrated under reduced pressure to obtain ethyl theanine of structural formula (II).
[0051] Step 2: Preparation of tea ethyl aromatic acid (TEC)
[0052] 21g of ethyl theanine was dissolved in 2.1L of anhydrous dichloromethane, 28g of 3-carboxycoumarin was added, and 0.21L of DIPEA (diisopropylethylamine) and 77g of EDCI were added respectively. The mixture was stirred at room temperature for 1.5 hours, then concentrated under reduced pressure, the solvent wa...
Embodiment 3
[0054] The difference between this embodiment and embodiment 2 is that
[0055] Step 1: Preparation of ethyl theanine
[0056] Theanine is dissolved in ethanol at a ratio of 91g / L (referring to dissolving 91g of theanine per liter of ethanol), and then slowly adding thionyl chloride to the system with a volume ratio of 57ml (referring to the dissolving solution of theanine and ethanol mixed with two volume ratio of thionyl chloride), the mixture was stirred at room temperature for 2 hours, and the resulting mixture was concentrated under reduced pressure to obtain theanine ethyl ester of structural formula (II).
[0057] Step 2: Preparation of tea ethyl aromatic acid (TEC)
[0058] 22g of ethyl theanine was dissolved in 2.2L of anhydrous dichloromethane, 32g of 3-carboxycoumarin was added, and 0.22L of DIPEA (diisopropylethylamine) and 78g of EDCI were added respectively. The mixture was stirred at room temperature for 2 hours, then concentrated under reduced pressure, the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com