Electroplating liquid of multi-layer cyanide-free electroplated copper-tin alloy coating, electroplating technology and coin thereof
A technology for electroplating copper and tin alloys, applied in coins, clothing, jewelry, etc., can solve the problems of cyanide-free electroplating of brass tin, such as inability to plate thick plating and unevenness, and improve the plating environment, reduce management costs, and reduce The effect of environmental influence pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
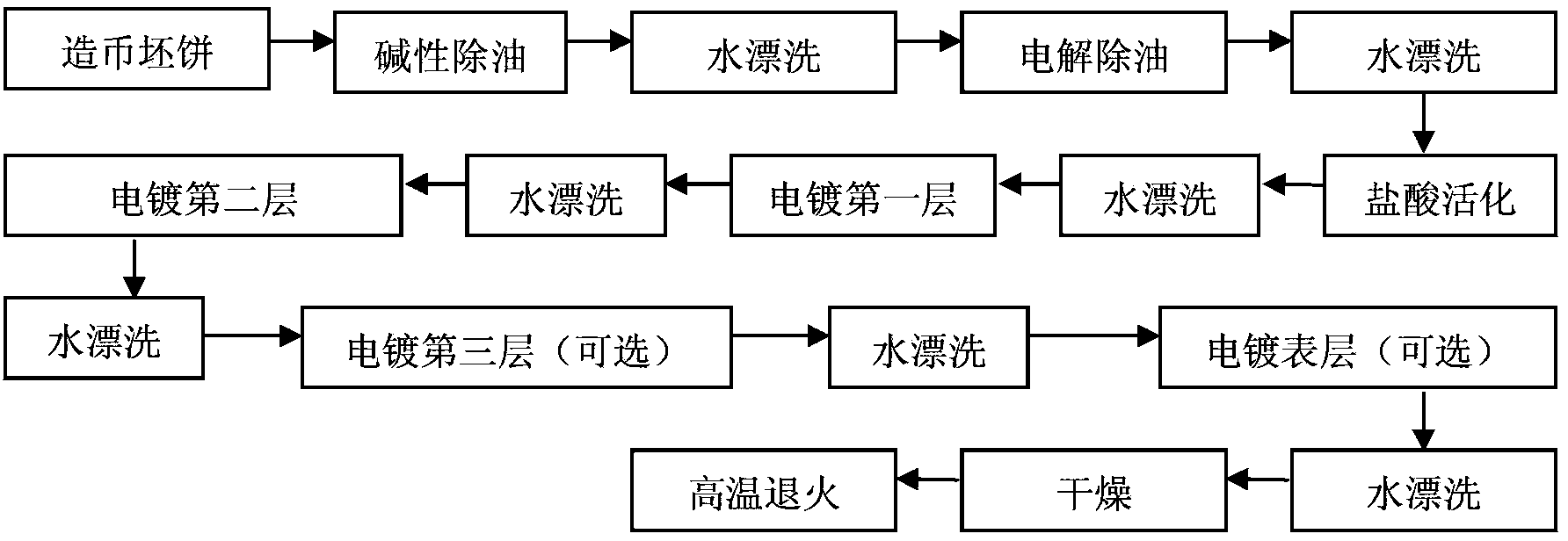
[0084] The low-carbon steel coinage blank is used as the substrate, the first layer and the second layer are electroplated sequentially on it, and then it is used as a product. The specific steps are as follows:
[0085] (1) Alkaline degreasing
[0086] Put the coinage blank into the alkaline degreasing liquid, the degreasing liquid is 50g / L degreasing agent, the temperature is 55°C for 20 minutes, and then rinse with 60°C deionized water;
[0087] (2) Electrodegreasing
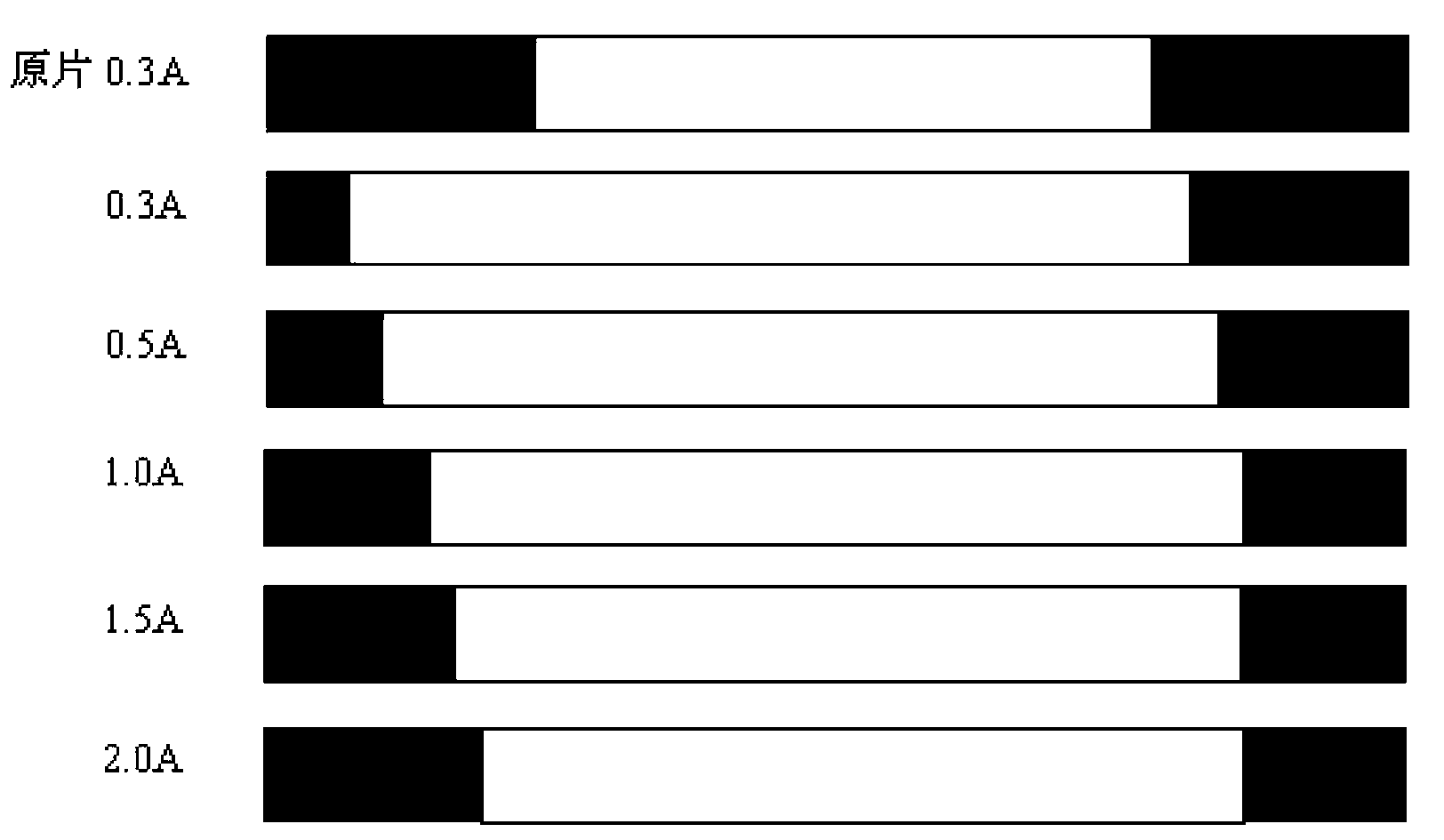
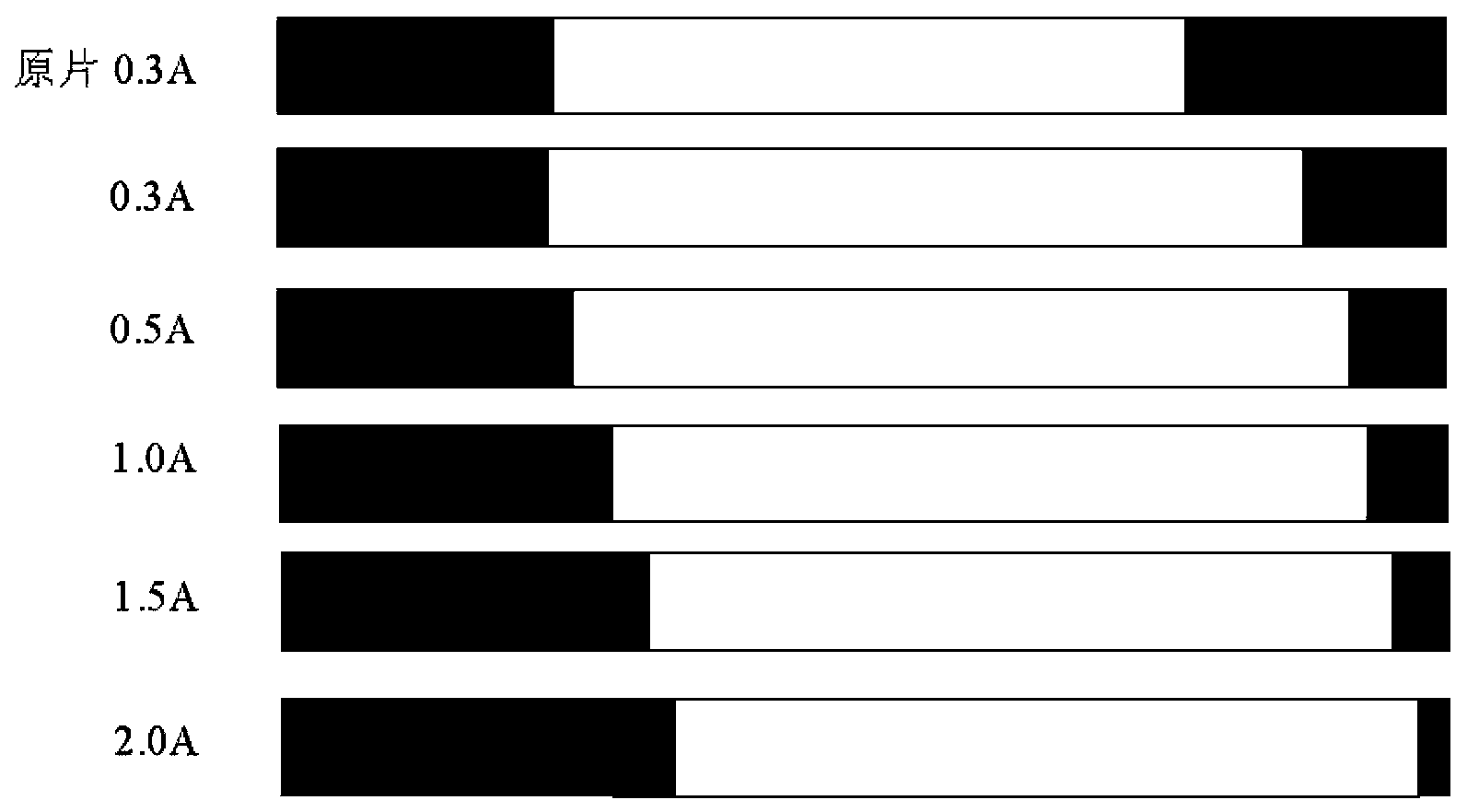
[0088] Put the above-mentioned coinage blanks after alkali washing in the electrolytic degreasing liquid, the degreasing liquid is 60g / L degreasing agent, the temperature is 55°C, and the current density is 0.5A / dm 2 , carry out anodic electrolytic cleaning for 20 minutes, and then rinse with deionized water at 60°C;
[0089] (3) Hydrochloric acid activation
[0090] Place the coinage blank after electrolytic degreasing in the HCL solution with a concentration of 350mL / L at a temperature of 20°C for 7 minu...
Embodiment 2
[0102] The low-carbon steel coinage blank is used as the substrate, the first layer and the second layer are electroplated sequentially on it, and then it is used as a product. The specific steps are as follows:
[0103] (1) Alkaline degreasing
[0104] Put the coinage blank into the alkaline degreasing liquid, the degreasing liquid is 60g / L degreasing agent, the temperature is 60°C for 20 minutes, and then rinse with 60°C deionized water;
[0105] (2) Electrodegreasing
[0106] Put the above-mentioned coinage blanks after alkali washing in the electrolytic degreasing liquid, the degreasing liquid is 70g / L degreasing agent, the temperature is 60°C, and the current density is 1.0A / dm 2 , carry out anodic electrolytic cleaning for 20 minutes, and then rinse with deionized water at 60°C;
[0107] (3) Hydrochloric acid activation
[0108] Place the coinage blank after electrolytic degreasing in the HCL solution with a concentration of 480mL / L at a temperature of 25°C for 7 minu...
Embodiment 3
[0120] The low-carbon steel coinage blank is used as the substrate, the first layer and the second layer are electroplated sequentially on it, and then it is used as a product. The specific steps are as follows:
[0121] (1) Alkaline degreasing
[0122] Put the mint blank into the alkaline degreasing liquid, the degreasing liquid is 70g / L degreasing agent, the temperature is 65°C for 20 minutes, and then rinse with 60°C deionized water;
[0123] (2) Electrodegreasing
[0124] Put the above-mentioned mint blanks after alkali washing in the electrolytic degreasing liquid, the degreasing liquid is 80g / L degreasing agent, the temperature is 65°C, and the current density is 1.2A / dm 2 , carry out anodic electrolytic cleaning for 20 minutes, and then rinse with deionized water at 60°C;
[0125] (3) Hydrochloric acid activation
[0126] Put the coinage blank after electrolytic degreasing in the HCL solution with a concentration of 480mL / L at a temperature of 29°C for 7 minutes of a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com