Human papilloma virus (HPV) resistant trivalent vaccine as well as preparation method and application thereof
A human papillomavirus, virus-like technology, applied in the fields of genetic engineering and vaccinology, can solve problems such as lack of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0184] Construction of high-efficiency expression system of HPV16L1 Pichia pastoris
[0185] 1. Optimization of HPV16L1 gene
[0186] Optimized sequence: According to the amino acid sequence of natural HPV16L1, the following factors are considered to optimize the design: the codon frequency used by Pichia pastoris, the G+C content in the gene and the secondary structure of mRNA.
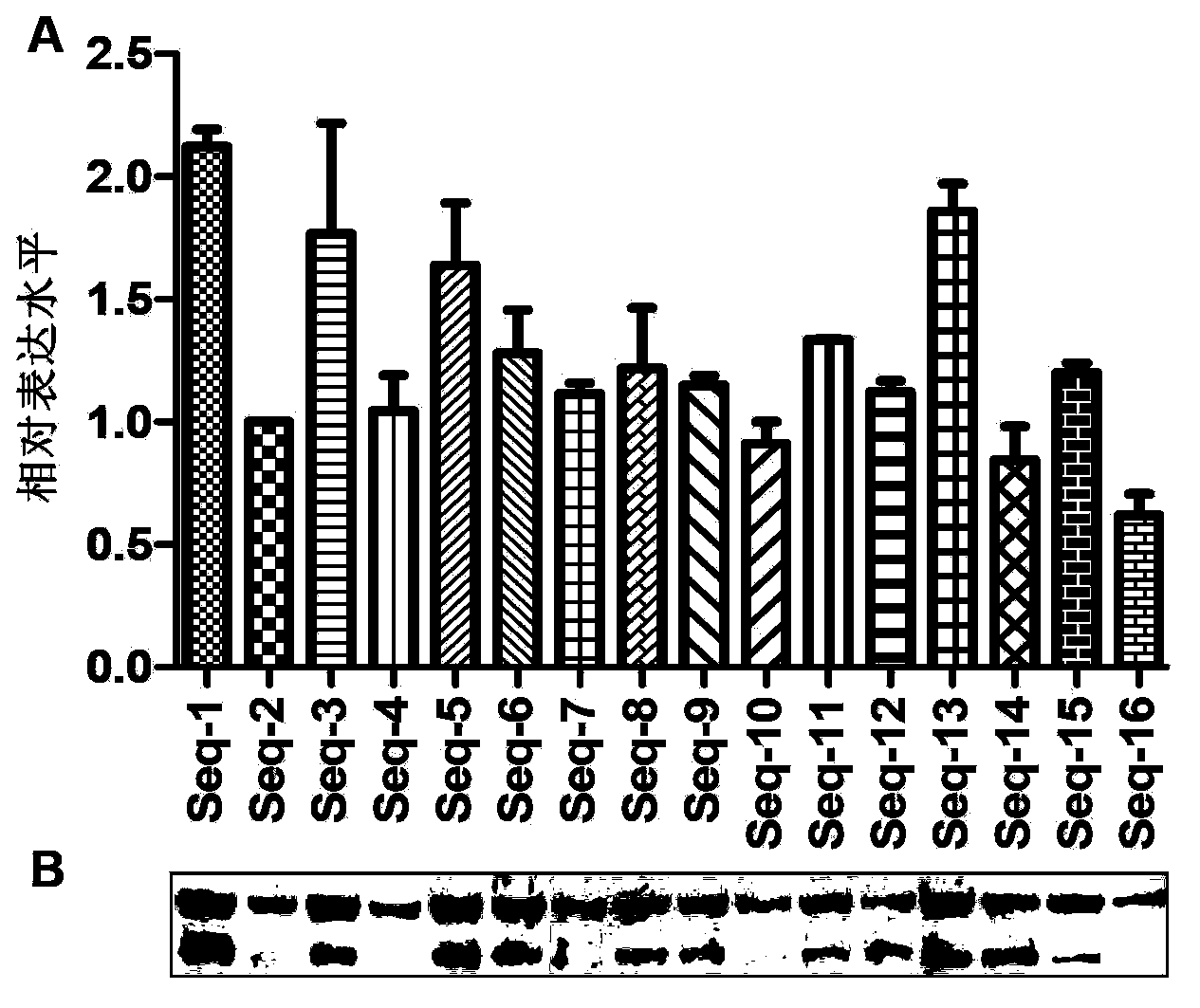
[0187] The optimized nucleotide sequence of this embodiment is shown in SEQ ID NO:3, and the amino acid sequence of the HPV16L1 protein encoded by the sequence is shown in SEQ ID NO:2.
[0188] The optimized gene (SEQ ID NO: 3) was artificially synthesized, and BamHI and EcoRI restriction sites were introduced at its 5' end and 3' end respectively; the control sequence 1 used the wild-type HPV16L1 gene (SEQ ID NO: 1), Encoding the same HPV16L1 protein, after comparison, the homology between the optimized nucleic acid sequence and the control wild-type gene sequence is about 77.0%
[0189] 2. Kozak...
Embodiment 2
[0201] Construction of High Expression System of HPV18L1 Pichia pastoris
[0202] 1. Optimization of the target gene
[0203] Optimized sequence: According to the amino acid sequence of natural HPV18L1, the following factors are considered to optimize the design: the codon frequency used by Pichia pastoris, the G+C content in the gene, and the secondary structure of mRNA.
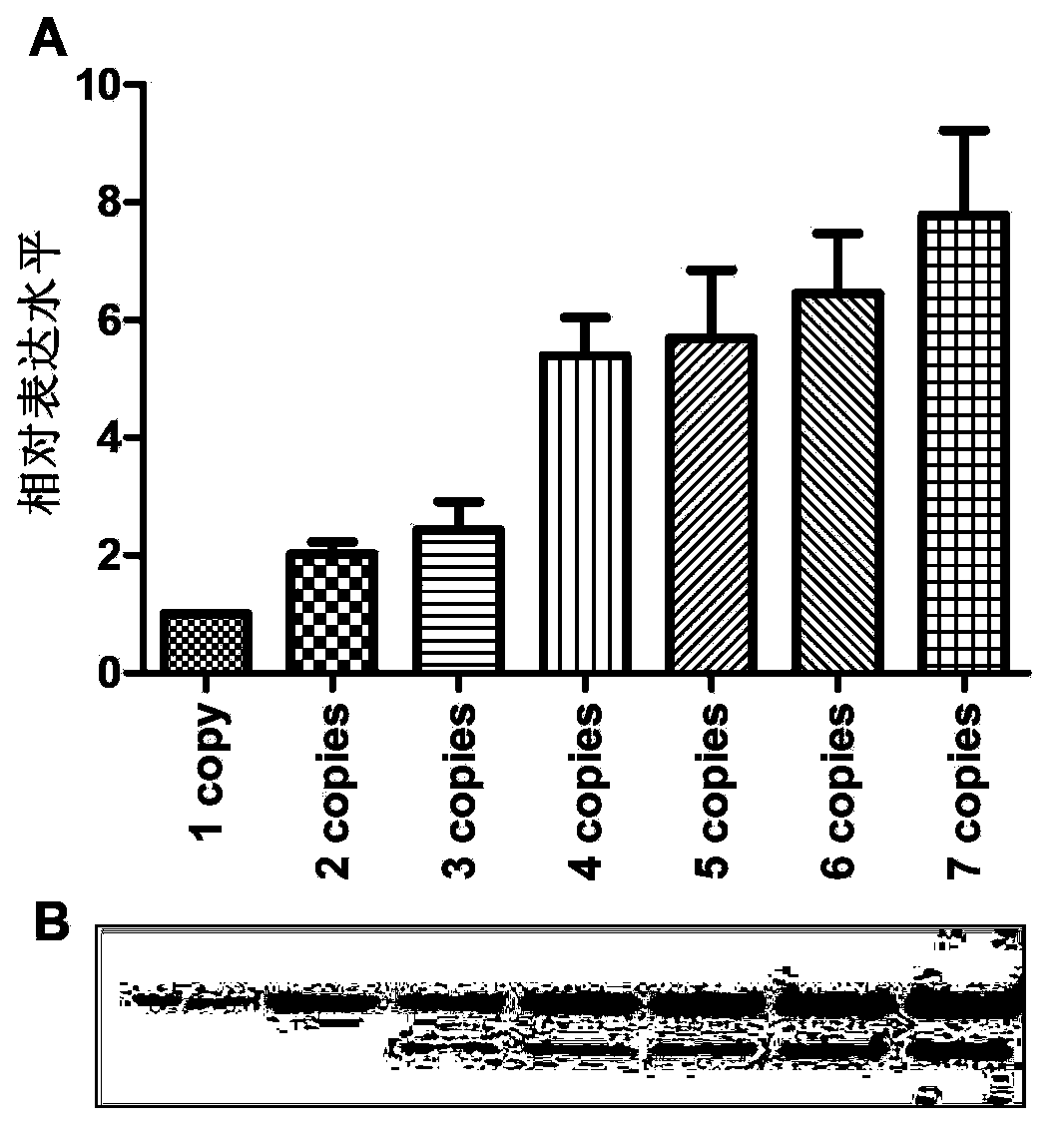
[0204] The nucleotide sequence of the target gene optimized in this example is shown in SEQ ID NO: 6, and the amino acid sequence of the HPV18L1 protein encoded by this sequence is identical to the natural sequence (shown in SEQ ID NO: 5). The optimized gene (SEQ ID NO: 6) was artificially synthesized, and BamHI and EcoRI restriction sites were introduced at its 5' end and 3' end respectively; the control sequence was the wild-type HPV18L1 gene (SEQ ID NO: 4), encoding For the same HPV18L1 protein, after comparison, the homology between the optimized sequence and the wild-type gene of the control is about...
Embodiment 3
[0211] Construction of High Expression System of HPV58L1 Pichia pastoris
[0212] Optimized sequence: According to the amino acid sequence of natural HPV58L1, the following factors are considered for optimal design: codon frequency used by Pichia pastoris, G+C content in gene, and secondary structure of mRNA.
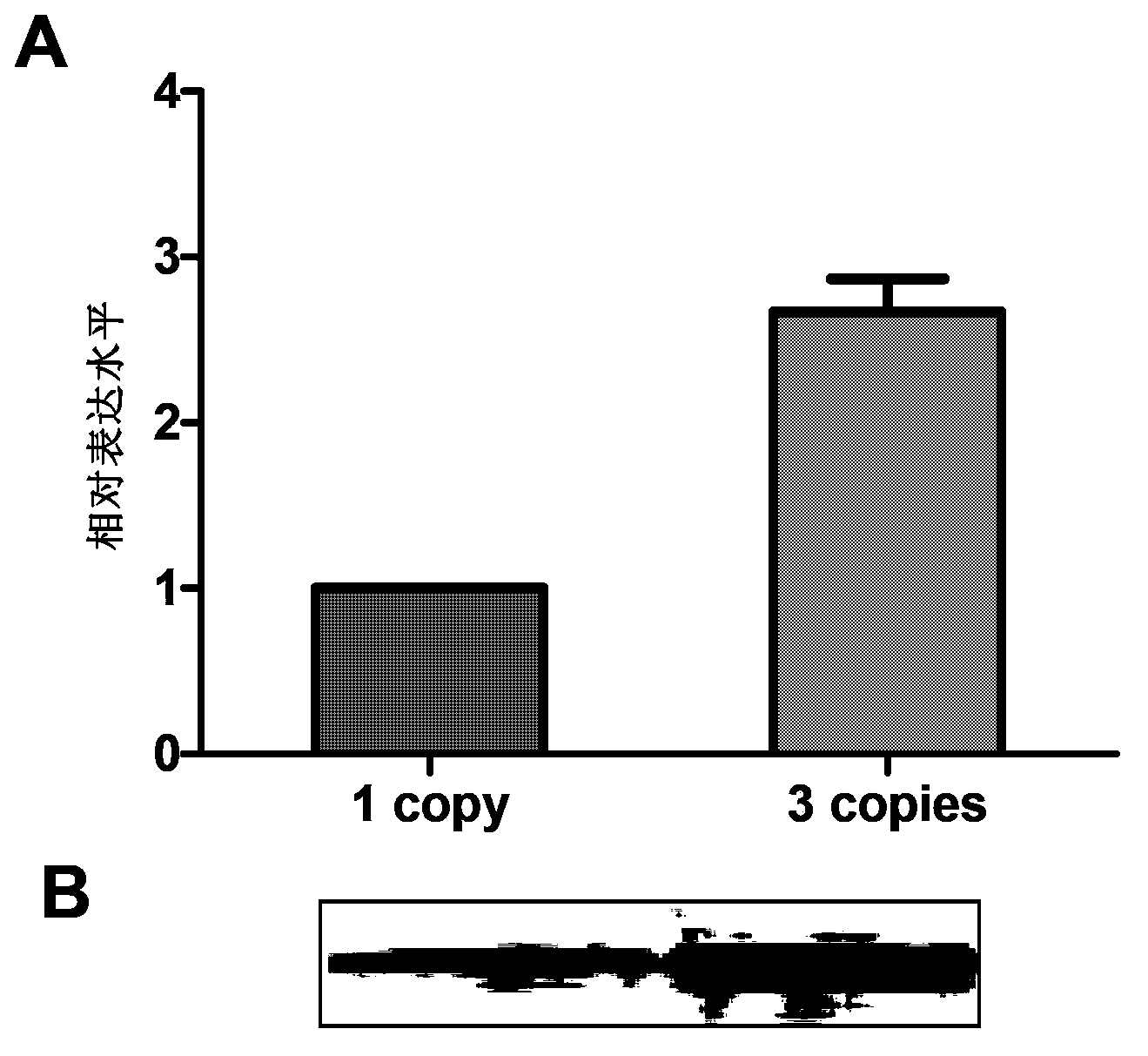
[0213] The optimized nucleotide sequence of this embodiment is shown in SEQ ID NO:9, and the amino acid sequence of the gene encoding HPV58L1 protein is shown in SEQ ID NO:8. The optimized gene (SEQ ID NO:9) was artificially synthesized, and BamHI and EcoRI restriction sites were introduced at its 5' end and 3' end, respectively.
[0214] Control sequence 1: wild-type HPV58L1 gene (SEQ ID NO: 7), encoding the same HPV58L1 protein; after comparison, the homology between the optimized sequence and the control wild-type gene is about 77.2%.
[0215] 2. Kozak sequence optimization
[0216] The optimized kozak sequence 1 screened in Example 1 was selected, and the identifi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com