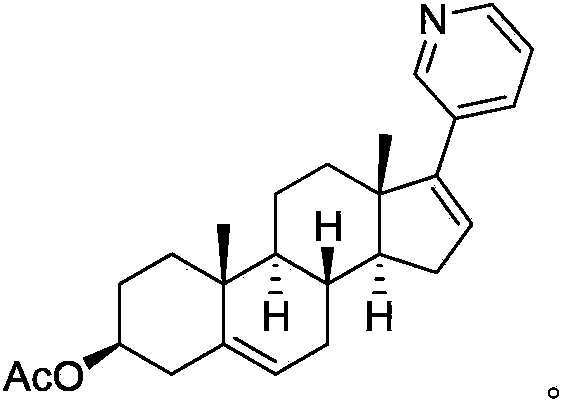
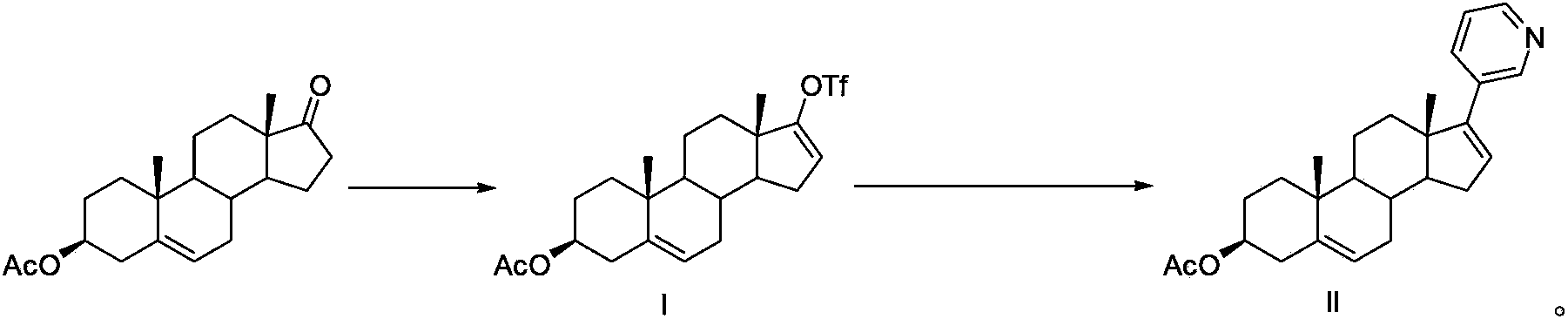
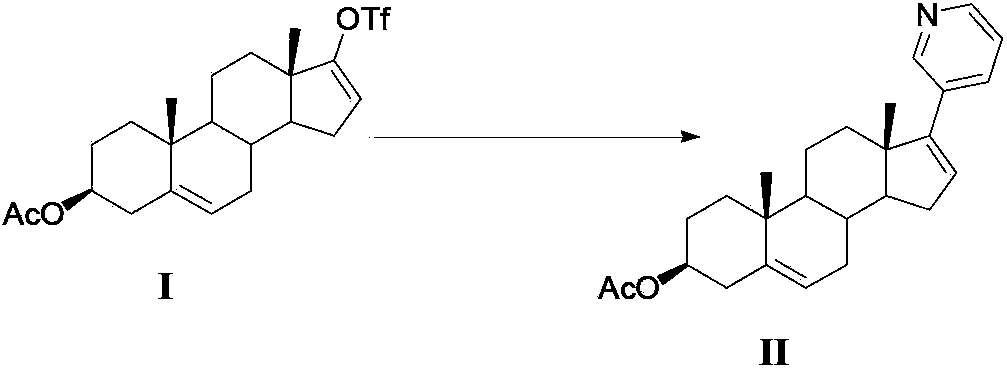
Method for preparing abiraterone acetate
A technology of abiraterone acetate and pyridine boric acid, applied in the directions of steroids, organic chemistry, etc., can solve the problems of increasing operation time and difficulty, unfavorable scale-up production, easy residual impurities, etc., achieves strong practical value, reduces raw material costs, The effect of easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Get 15g formula I compound, after dissolving with 100mL dioxane, transfer to a 250mL there-necked flask equipped with thermometer, reflux condenser and argon conduit; Under argon protection, add 3-pyridineboronic acid ( 4.8g), potassium carbonate aqueous solution (50mL) with a molar concentration of 2mol / L, and bis(triphenylphosphine)palladium dichloride (35mg); heated to reflux under stirring, and the reaction was completed (about 8 hours of reflux reaction), Add 50mL ethyl acetate and 50mL water to the reaction system, separate the liquid, extract the water phase with 3×50mL ethyl acetate; collect the organic phase, wash with 2×50mL water first, then wash with 2×50mL saturated brine; Dry over sodium sulfate, filter, and concentrate the organic solvent in the dry filtrate to obtain 15 g of brown oil; add ethyl acetate-n-hexane mixed solvent with a volume ratio of 1:4 to this oil, and stir at room temperature for 3 hours to make After full analysis and cryst...
Embodiment 2
[0034] Get 15g formula I compound, after dissolving with 50mL toluene, transfer to a 250mL there-necked flask equipped with thermometer, reflux condenser and argon conduit; ), a potassium carbonate aqueous solution (50mL) with a molar concentration of 2mol / L and bis(triphenylphosphine)palladium dichloride (35mg); heated to reflux under stirring, and the reaction was completed (about 8 hours of reflux reaction), to the reaction Add 50mL ethyl acetate and 50mL water to the system, separate the liquids, and extract the aqueous phase with 3×50mL ethyl acetate; collect the organic phase, wash it with 2×50mL water first, and then wash it with 2×50mL saturated brine; anhydrous sulfuric acid Dry over sodium, filter, and concentrate the organic solvent in the dry filtrate to obtain 14 g of brown oil; add ethyl acetate-n-hexane mixed solvent with a volume ratio of 1:4 to this oil, stir at room temperature for 3 hours to fully analyze Crystallization, to obtain 7.5g of dark gray solid, m...
Embodiment 3
[0036]Take 15g of the compound of formula I, dissolve it with 90mL of tetrahydrofuran, and transfer it to a 250mL three-neck flask equipped with a thermometer, reflux condenser and argon gas conduit; under the protection of argon, add 3-pyridineboronic acid pinacol ester to the system (8.2g), dipotassium hydrogen phosphate aqueous solution (50mL) with a molar concentration of 2mol / L, and tetrakis(triphenylphosphine)palladium (30mg); heated to reflux under stirring, and the reaction was completed (about 8 hours of reflux reaction), Add 50mL ethyl acetate and 50mL water to the reaction system, separate the liquid, extract the water phase with 3×50mL ethyl acetate; collect the organic phase, wash with 2×50mL water first, then wash with 2×50mL saturated brine; Dry over sodium sulfate, filter, and concentrate the organic solvent in the dry filtrate to obtain 14.5 g of brown oil; add ethyl acetate-n-hexane mixed solvent with a volume ratio of 1:4 to this oil, and stir at room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com