Mixed-ligand porous aluminum metal organic framework material and preparation method thereof
A technology of metal-organic frameworks and mixed ligands, applied in organic chemistry, chemical instruments and methods, and other chemical processes, can solve unseen problems and achieve low cost, high yield, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
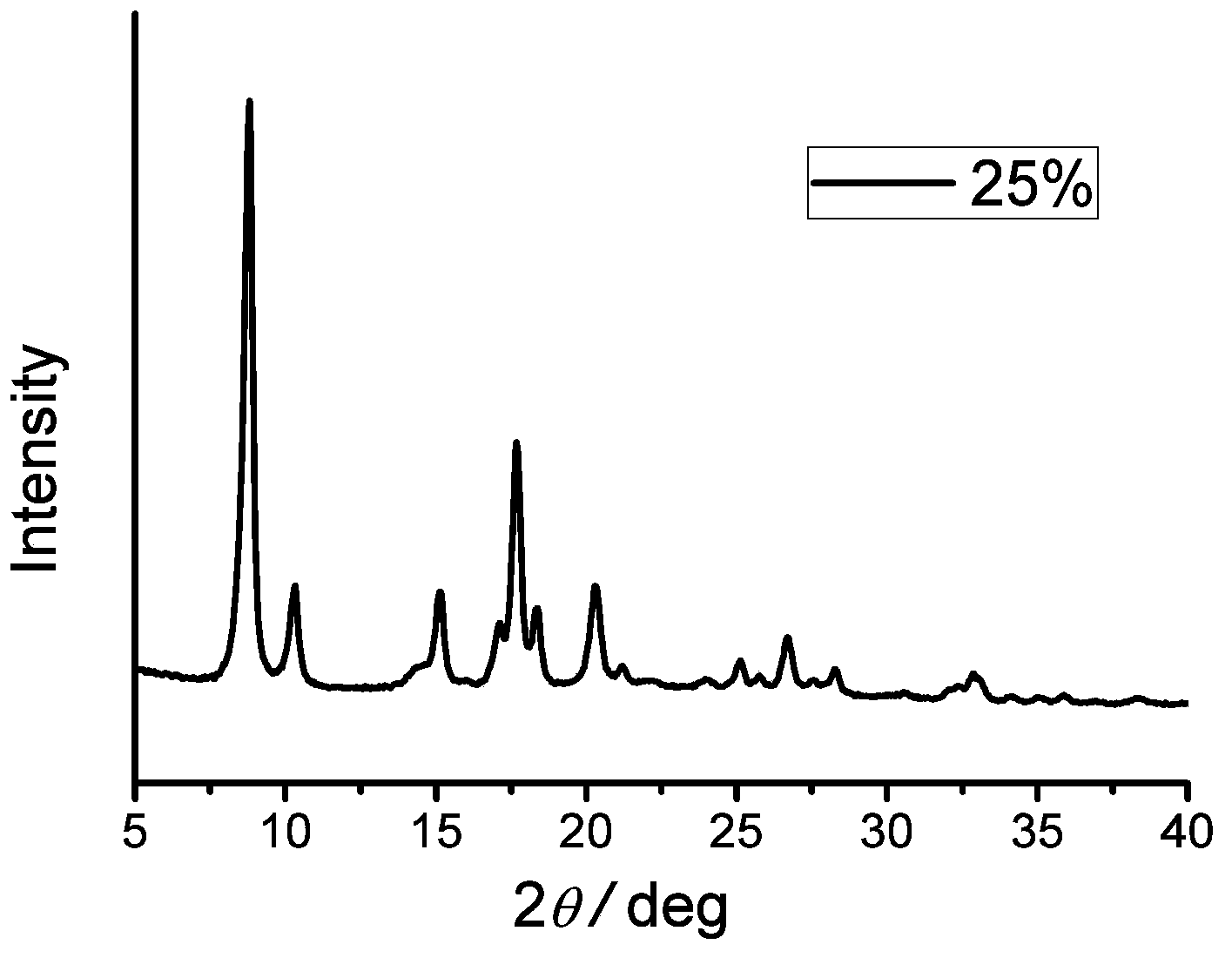
[0031] 1) Weigh 7.5g of aluminum nitrate and 1.08g of 1,4-naphthalene dicarboxylic acid / 2.72g of 2-aminoterephthalic acid (molar ratio: 5:15, the addition percentage is 25%) and dissolve in 195ml N,N- Dimethylformamide was fully dissolved by magnetic stirring, and then the mixed solution was transferred to three 100ml stainless steel reaction kettles lined with polytetrafluoroethylene, reacted in a synthesis oven at 220°C for 3 days, and cooled naturally to room temperature.
[0032] 2) The product was suction filtered, washed with N,N-dimethylformamide, dried at 50°C, and finally calcined at 200°C for 8 hours to obtain the target product.
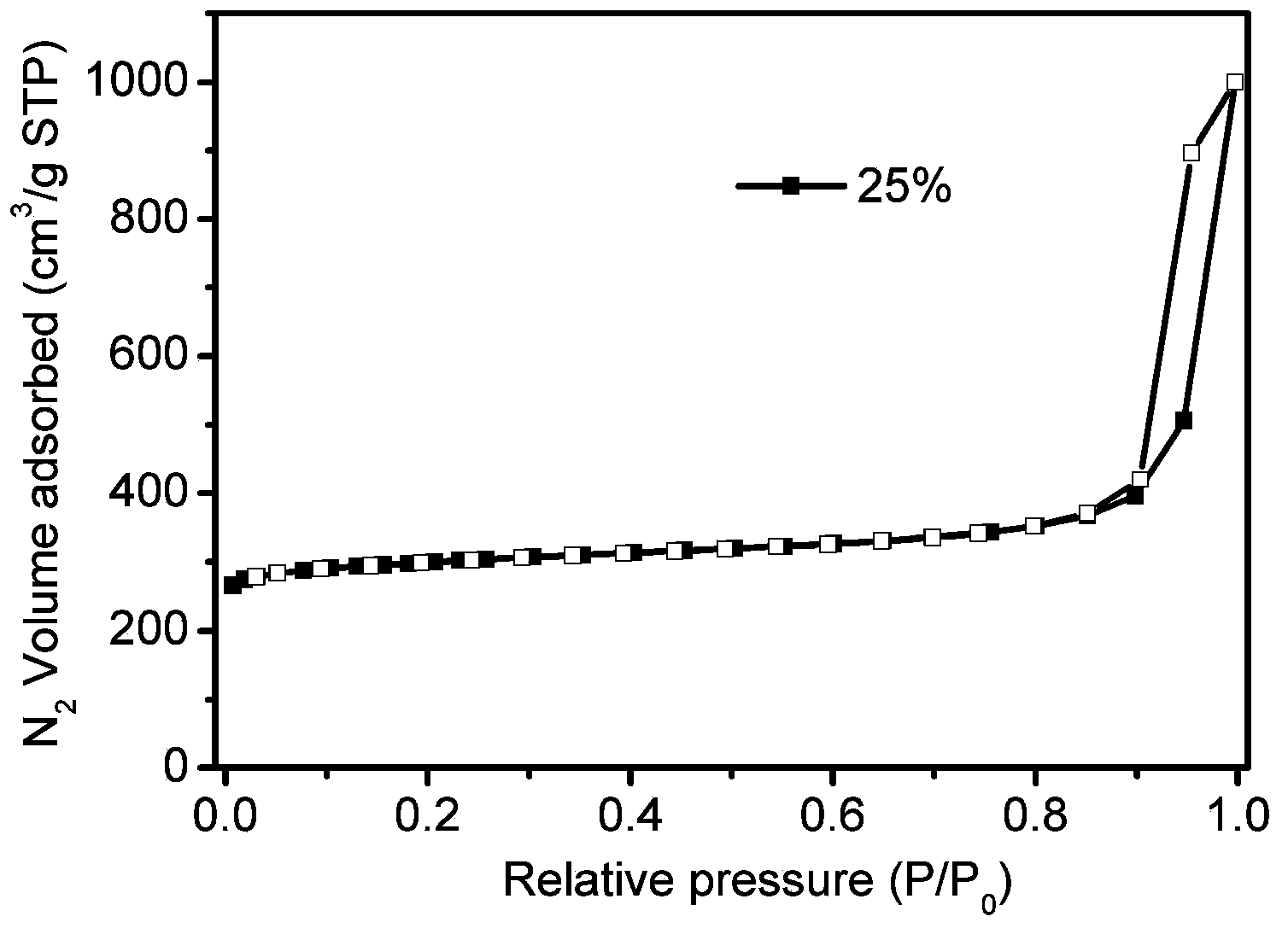
[0033] 3) Gas isothermal adsorption performance test: the treatment condition is degassing at 200°C for 24 hours. The test condition is 77K, isothermal adsorption of nitrogen and hydrogen; carbon dioxide adsorption test at 273K.
Embodiment 2
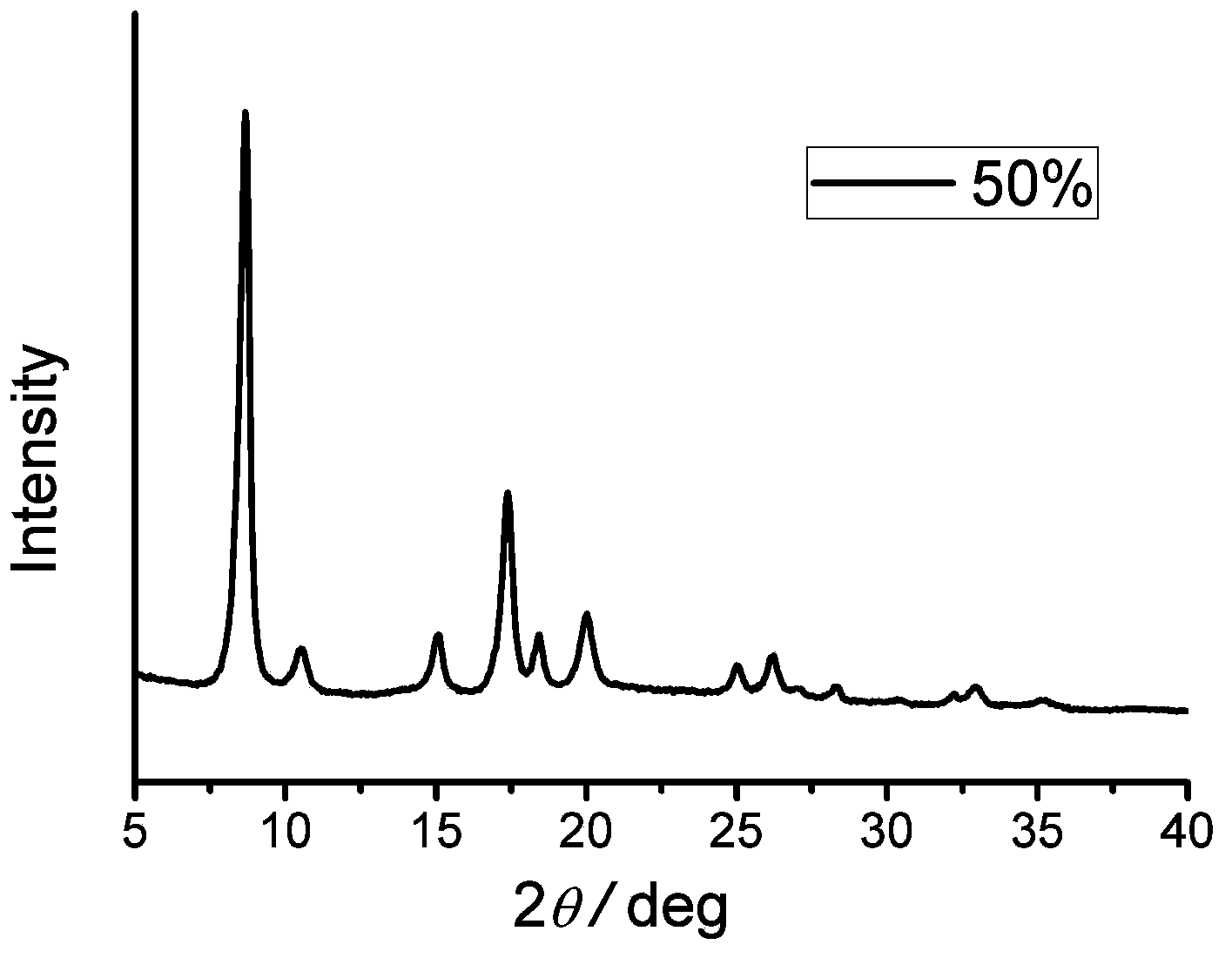
[0035] 1) Weigh 7.5g of aluminum nitrate and 2.18g of 1,4-naphthalene dicarboxylic acid / 1.82g of 2-aminoterephthalic acid (molar ratio: 10:10, the percentage added is 50%) and dissolve in 195ml of N,N- Dimethylformamide was fully dissolved by magnetic stirring, and then the mixed solution was transferred to three 100ml stainless steel reaction kettles lined with polytetrafluoroethylene, reacted in a synthesis oven at 220°C for 3 days, and cooled naturally to room temperature.
[0036] 2) The product was suction filtered, washed with N,N-dimethylformamide, dried at 50°C, and finally calcined at 200°C for 8 hours to obtain the target product.
[0037] 3) Gas isothermal adsorption performance test: the treatment condition is degassing at 200°C for 24 hours. The test conditions are 77K, isothermal adsorption of nitrogen and hydrogen; carbon dioxide adsorption test at 273K.
Embodiment 3
[0039] 1) Weigh 7.5g of aluminum nitrate and 1.61g of 1,4-naphthalene dicarboxylic acid / 0.91g of 2-aminoterephthalic acid (molar ratio: 15:5, the percentage added is 75%) and dissolve in 195ml of N,N-dimethyl base formamide, magnetically stirred to make it fully dissolved, and then the mixed solution was transferred to three 100ml stainless steel reaction kettles lined with polytetrafluoroethylene, reacted in a synthesis oven at 220°C for 3 days, and naturally cooled to room temperature.
[0040] 2) The product was suction filtered, washed with N,N-dimethylformamide, dried at 50°C, and finally calcined at 200°C for 8 hours to obtain the target product.
[0041] 3) Gas isothermal adsorption performance test: the treatment condition is degassing at 200°C for 24 hours. The test conditions are 77K, isothermal adsorption of nitrogen and hydrogen; carbon dioxide adsorption test at 273K.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com