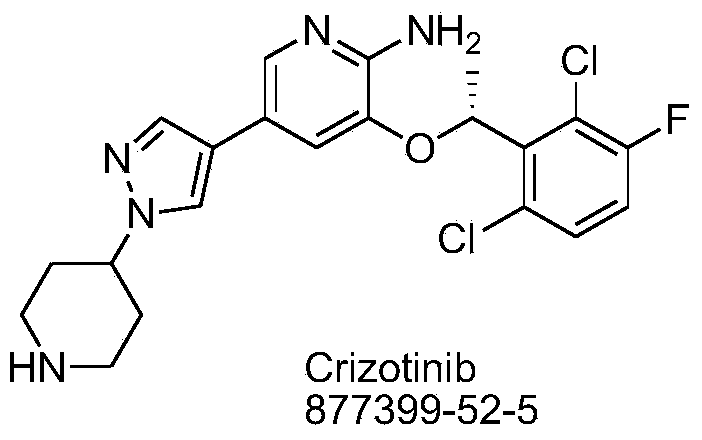
Synthetic process method for novel antineoplastic molecular targeted drug of crizotinib
An anti-tumor molecule, crizotinib technology, applied in organic chemistry methods, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve problems such as unsatisfactory yield and waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0095] Example 1: Synthesis of racemic 1-(2,6-dichloro-3-fluorophenyl)ethanol (TM1)
[0096] In a 5L three-neck flask, add 832 grams of SM1 to a mixed solvent of 1.5L ethanol and 1.5L THF, heat the solution to 35°C, add 182 grams of sodium borohydride, the reaction is exothermic, and bubbles are generated, and the reaction is completed after 2 hours. Add 50 g of sodium borohydride, and TLC detects that the reaction is complete in 2 hours (PE / EA=10:1). Add 200 g of water to the system, stir for half an hour, a large amount of solids appear, remove the solvent under reduced pressure, add water and ethyl acetate for extraction, wash the ethyl acetate layer with salt, dry and spin dry to obtain 800 g of product TM1, the yield is 97%. 1 H NMR (400MHz, DMSO-d 6 )δppm7.42(m,1H),7.32(m,1H),5.42(m,2H),1.45(d,J=6.4Hz,3H).
example 2
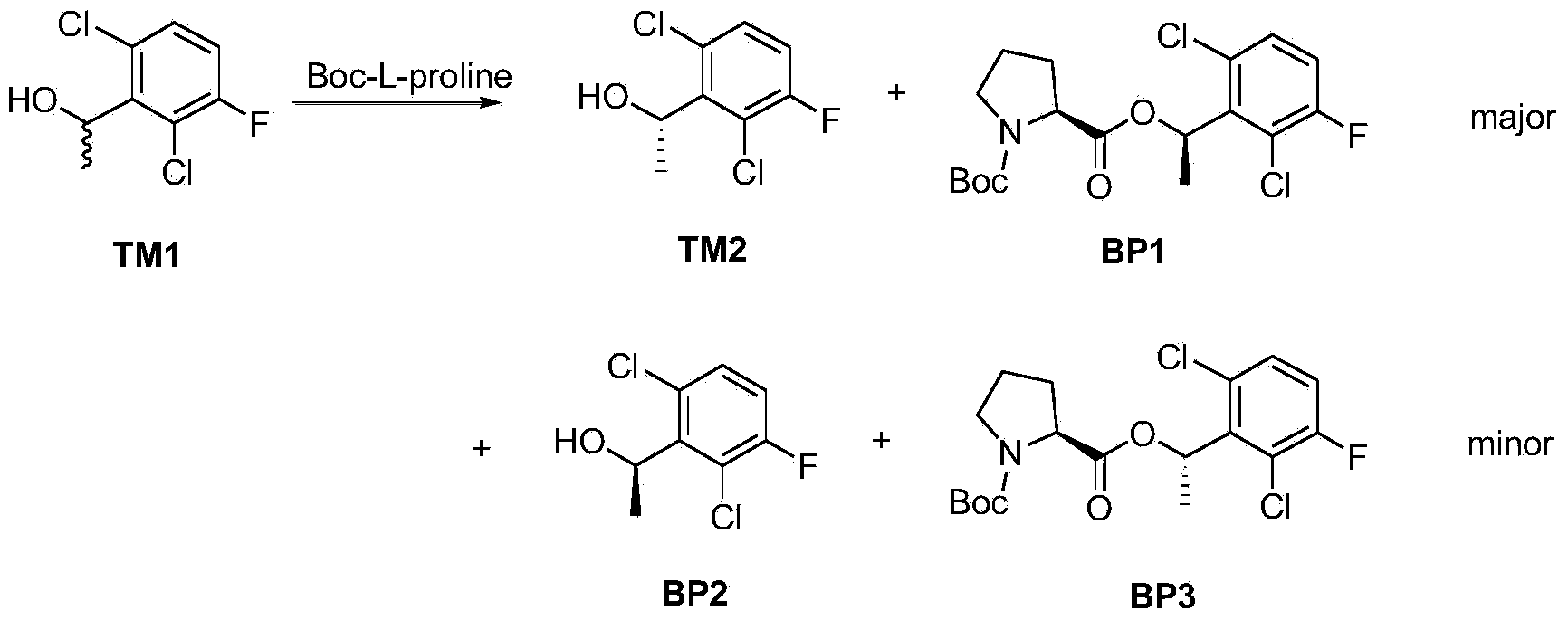
[0097] Example 2: Preparation of (S)-1-(2,6-dichloro-3-fluorophenyl)ethanol (TM2)
[0098] 500 grams of TM1, 322 grams of Boc-L-proline were dissolved in 1.5L of anhydrous dichloromethane, and 2.5L of dichloromethane solution of 375 grams of EDCI and 118 grams of p-toluenesulfonic acid was added dropwise at 0°C, and the addition was completed Afterwards, the reaction was carried out at room temperature for 2 hours, and the reaction was monitored by TLC (PE / EA=5:1). The reaction solution was washed with water, dried, and evaporated to dryness. The fraction at 100-110°C was collected under reduced pressure with an oil pump at a vacuum of 10 mm Hg to obtain an oily crude product. The crude product was dissolved in 250 mL of n-hexane at room temperature, and the product precipitated when the temperature dropped to -20°C. Filtration at low temperature yielded 150 g of a white solid, with a yield of 60% based on the single configuration and 30% based on the total amount (ee%>98%). ...
example 3
[0108] Example 3: Synthesis of (R)-3-[1-(2,6-dichloro-3-fluorophenyl)ethoxy]-2-nitropyridine (TM3)
[0109] 300 grams of TM2, 205 grams of SM2 and 540 grams of triphenylphosphine were dissolved in 2L THF, and 361 grams of DEAD was added dropwise at 0°C. After the addition was completed, it was stirred at room temperature for two hours, and the reaction was detected by TLC. (PE / EA =3:1). Remove THF by rotary evaporation, add water, extract with ethyl acetate 1Lx3, wash the ethyl acetate layer with saturated aqueous sodium bicarbonate solution, dry the ethyl acetate layer and concentrate to 2L, cool down to 0°C to precipitate a large amount of solids, remove by filtration, and concentrate the filtrate to 1L, solid precipitated after cooling down, filtered, combined the solids obtained by the two filtrations, washed with PE / EA=5:1 solution, filtered, removed the solid (about 500g), combined the filtrates and removed the solvent under reduced pressure to obtain a crude product. 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com