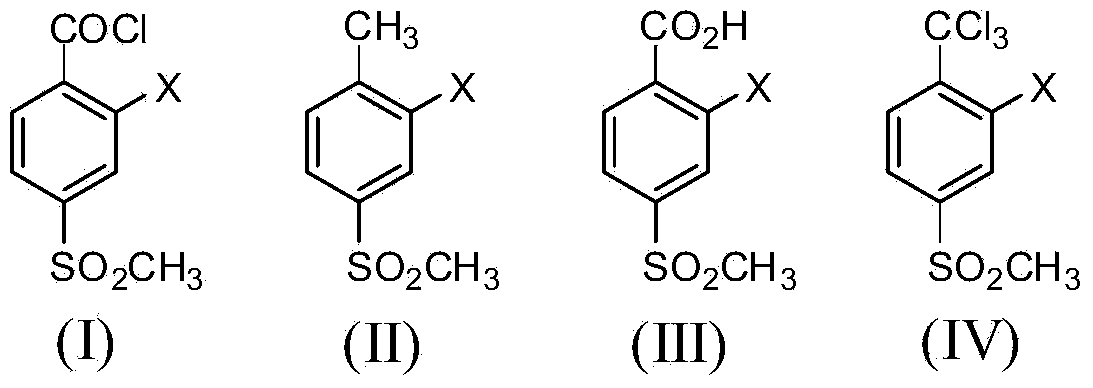
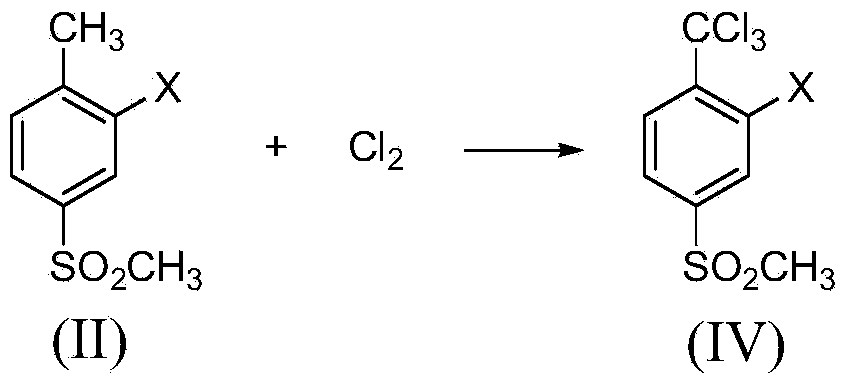2-substituent-4-methylsulfonyl-alpha,alpha,alpha-trichlorotoluene, and preparation method and application thereof
A technology of thiamphenyltoluene and trichlorotoluene, which is applied in the field of triketone herbicide intermediates, achieves the effects of low cost, short process route, and less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
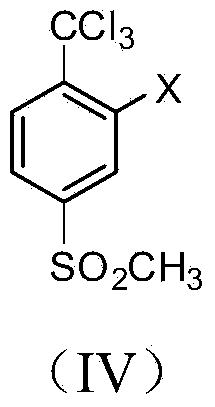
[0023] Example 1: Synthesis of 2-chloro-4-thiamphenicol-α, α, α-trichlorotoluene
[0024] Add 2-chloro-4-thiamphenicol toluene (40.9g, 0.2mol) and 205mL carbon tetrachloride into a 500mL tetrafluoro-lined autoclave, stir and dissolve at room temperature, then add sodium carbonate (63.6g, 0.6mol), and then Pass in chlorine gas (42.6g, 0.6mol), raise the temperature to 60°C and stir for 12 hours, the reaction pressure is 0.2-0.6MPa, cool to room temperature after the reaction, ventilate excess chlorine gas, take out the reaction solution, filter, add 50mL water to the filtrate and wash , layered, and the organic layer was dried with anhydrous sodium sulfate and then precipitated under reduced pressure to obtain 60.5 g of a brownish-yellow liquid. Through gas chromatography analysis, the content of 2-chloro-4-thiamphenicol-α,α,α-trichlorotoluene It was 92.3%, and the yield was 90.6%. MS(m / e): 306(76%), 308(100%), 310(47%). 1 H NMR(δ,ppm):7.96(d,J=2.0Hz,1H),7.82(dd,J=8.0Hz,2.0Hz...
Embodiment 2
[0025] Example 2: Synthesis of 2-chloro-4-thiamphenicol-α, α, α-trichlorotoluene
[0026] Add 2-chloro-4-thiamphenicol toluene (40.9 g, 0.2 mol) and 327 mL of carbon tetrachloride into a 1000 mL tetrafluoro-lined autoclave, stir and dissolve at room temperature, then add potassium carbonate (110.4 g, 0.8 mol), and then Pass in chlorine gas (56.8g, 0.8mol), raise the temperature to 80°C and stir for 8 hours, and the reaction pressure is 0.4-1.0MPa. After the reaction, cool to room temperature, ventilate excess chlorine gas, take out the reaction liquid, filter, and add 80 mL of water to the filtrate for washing , layered, and the organic layer was desolvated under reduced pressure to obtain 61.2 g of a brownish-yellow liquid. According to gas chromatography analysis, the content of 2-chloro-4-thiamphenicol-α, α, α-trichlorotoluene was 98.4%, and the yield was 97.8% %.
Embodiment 3
[0027] Example 3: Synthesis of 2-nitro-4-thiamphenicol-α, α, α-trichlorotoluene
[0028] Add 2-nitro-4-thiamphenicol toluene (43.0g, 0.2mol) and 258mL p-chlorobenzotrifluoride into a 500mL PTFE-lined autoclave, stir and dissolve at room temperature, then add sodium carbonate (74.2g, 0.7mol) , then pass in chlorine gas (49.7g, 0.7mol), heat up to 80°C and stir for 12 hours, the reaction pressure is 0.2-0.8MPa, after the reaction is completed, cool to room temperature, ventilate the excess chlorine gas, take out the reaction liquid, filter, add water to the filtrate Wash with 50 mL, separate layers, and desolvate the organic layer under reduced pressure to obtain 61.5 g of brown-yellow liquid. According to gas chromatography analysis, the content of 2-nitro-4-thiamphenicol-α,α,α-trichlorotoluene is 96.6%. The yield is 93.3%. MS(m / e): 317(100%), 319(95%), 321(30%). 1 H NMR(δ,ppm):8.34(d,J=2.0Hz,1H),8.32(dd,J=7.6Hz,2.0Hz,1H),7.99(d,J=7.6Hz,1H),3.28(s ,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com