Gene knock-in recombinant vector and preparation method thereof as well as method for preparing mouse model
A recombinant vector and gene knock-in technology, applied in the biological field, can solve the problems of inconvenient and flexible operation, high requirements for experimental instruments and equipment, and low fluorescence sensitivity, and achieve the effects of clean background, accurate experimental results, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
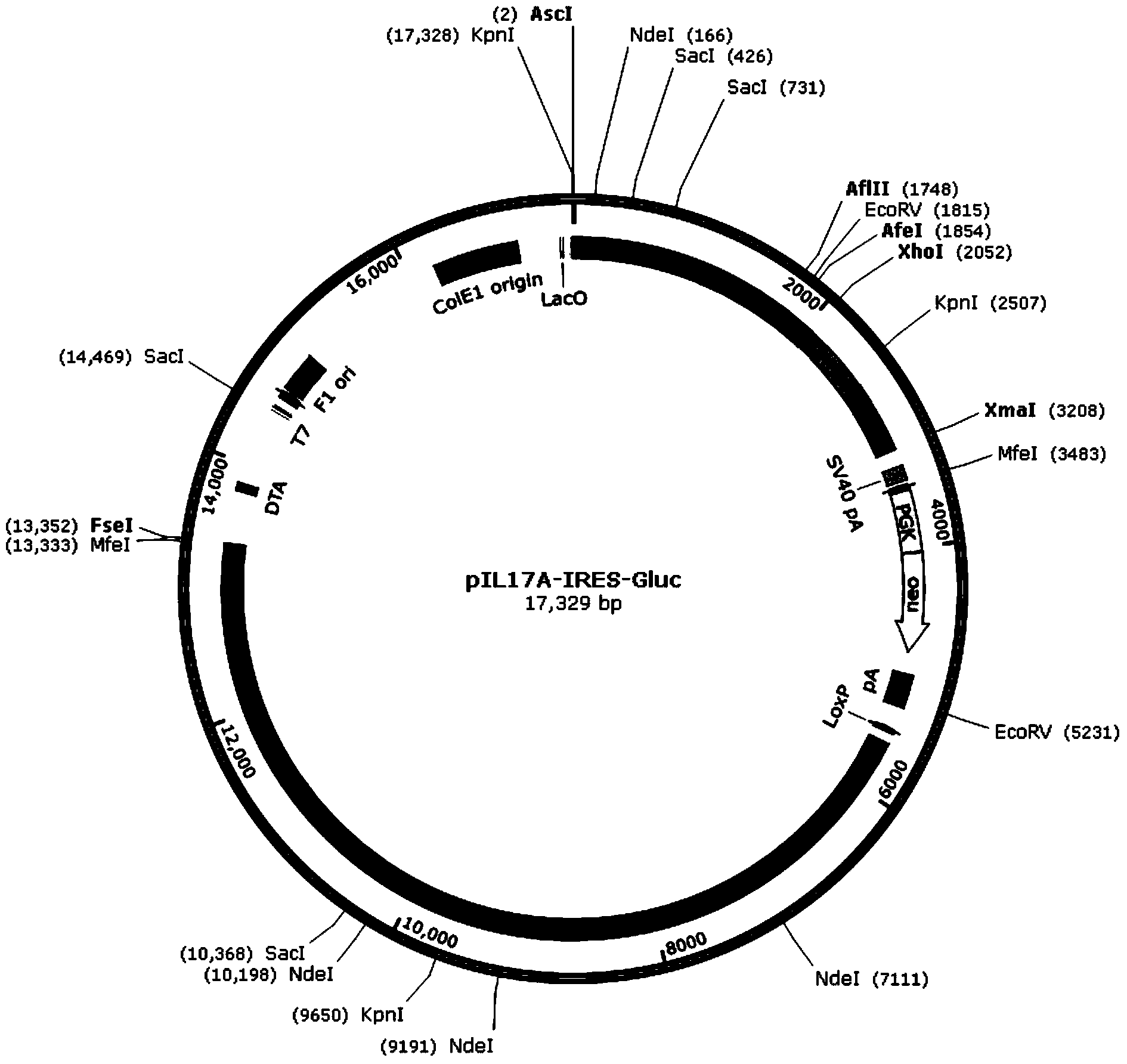
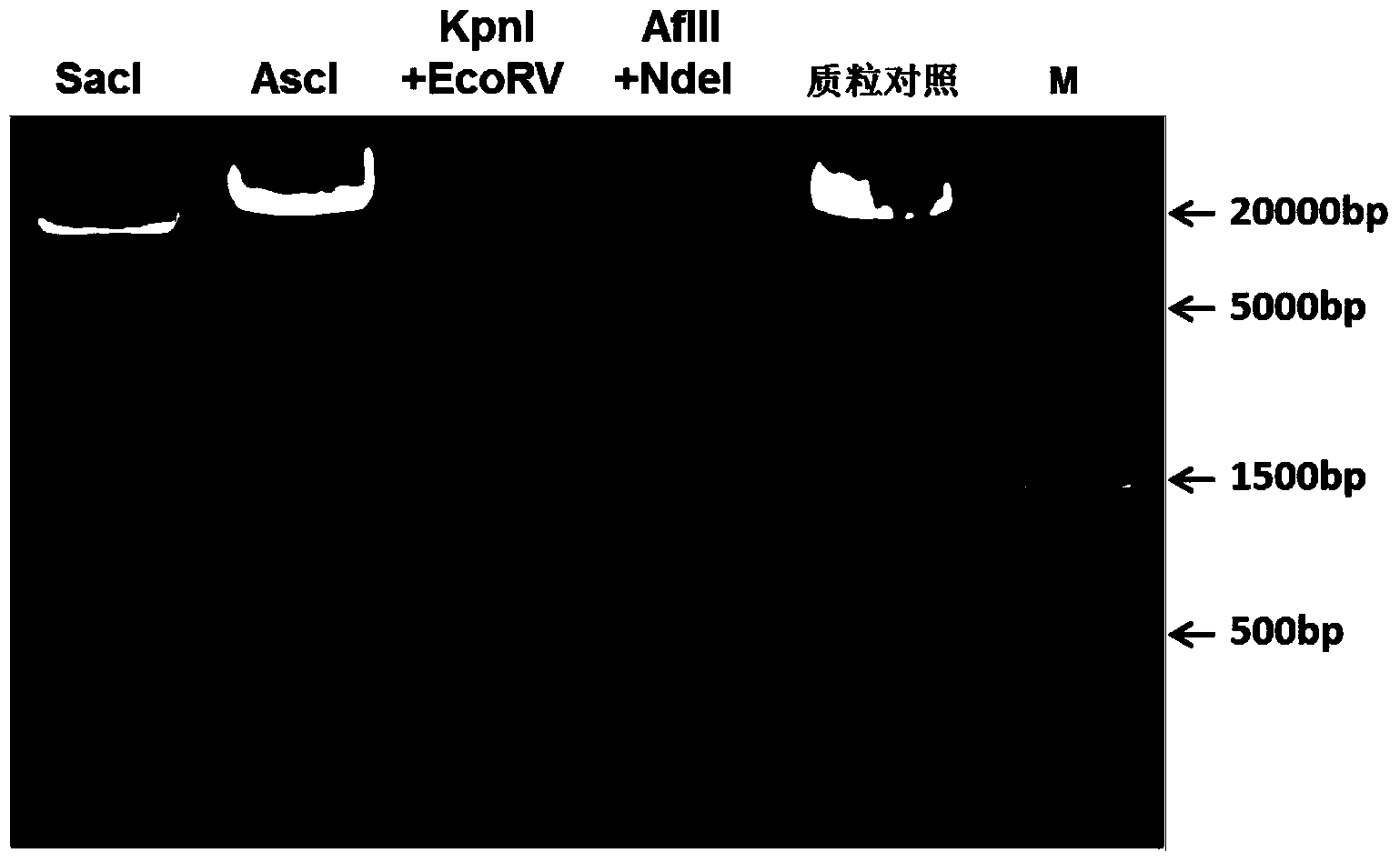
[0033] The present invention also provides a method for preparing the recombinant vector, wherein the method includes introducing the DNA fragment structure IRES-luc into the expression vector pIL17A-GFP. For example, primers can be designed according to IRES, luc and the cloning site in the vector, the IRES and luc sequences can be obtained by PCR, the IRES and luc sequences can be purified and recovered, and the DNA fragment structure IRES-luc can be obtained by PCR connection, and then double enzyme digestion method can be used to The DNA fragment structure IRES-luc was inserted between the cloning sites XhoI and XmaI in the expression vector to obtain a recombinant vector.
[0034] In a specific embodiment of the present invention, the preparation method may include the following steps: design primers according to IRES, Gluc and the cloning site in the vector, and obtain IRES and Gluc sequence, purification and recovery of IRES and Gluc sequences and PCR connection to obta...
Embodiment 1
[0066] This example is used to illustrate the construction method of the gene knock-in vector provided by the present invention.
[0067] 1. Amplify the coding sequence of IRES and the coding sequence of luciferase Gluc by PCR reaction respectively, and the primers used are shown in Table 1.
[0068] (1) Primers:
[0069] Table 1
[0070] Primer name
nucleic acid sequence
IRES-F
5'-atctctcgaggttaacgaattccgccccccccccctaacgtta-3'
IRES-R
5'-gaactttgactcccatggttgtggccatattatcatcgtgtttttcaaag-3'
Gluc-F
5'-ctttgaaaaacacgatgataatatggccacaaccatgggagtcaaagttc-3'
Gluc-R
5'-acatcccgggcttagtcaccacc-3'
[0071] in,
[0072] The primer pair IRES-F and IRES-R are used to amplify the coding nucleotide sequence of the IRES gene from the pT7CFE1 plasmid;
[0073] The primer pair Gluc-F and Gluc-R was used to amplify the coding nucleotide sequence of the Gluc gene from the pMCS-Gluc plasmid.
[0074] The primer pair IRES-F and G...
Embodiment 2
[0090] This embodiment is used to illustrate the preparation method of the mouse model provided by the present invention:
[0091] (1) Culture, transfection and positive clone screening of embryonic stem cells:
[0092] 1. Culture of embryonic stem cells
[0093] C57BL / 6 embryonic stem cells were cultured in a culture dish covered with feeder cells, and placed in an incubator at 37°C, 5% CO2, and saturated humidity. The composition of the medium used is as follows in Table 2:
[0094] Table 2
[0095] Medium composition
volume
Knockout DMEM
500ml
FBS
90ml
MEM NEAA
6ml
6ml
ESGRO LiF
60μL
β-mercaptoethanol
600μL
[0096] 2. Electroporation transfection
[0097] After the 100mm culture dish full of cells is taken out from the CO2 incubator, the stem cell culture medium of the 100mm dish is aspirated. Add 5ml of PBS along the wall of each dish, shake it gently, suck it out, and w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com