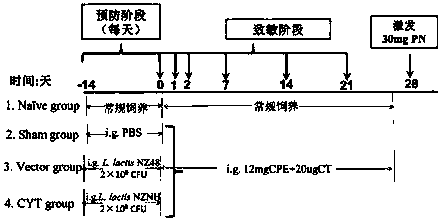
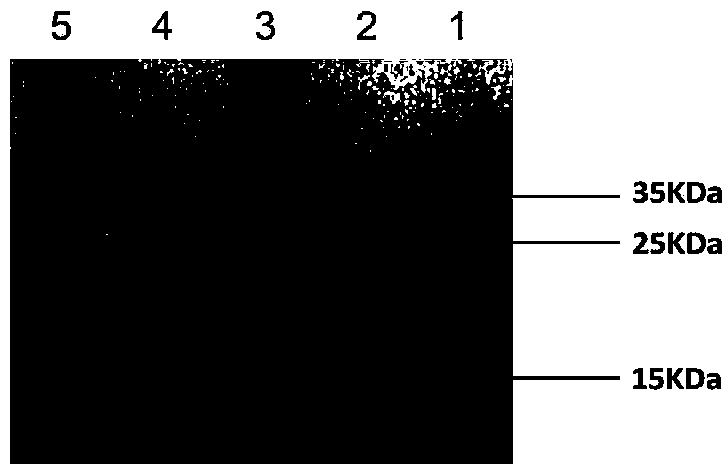
Recombinant lactococcus lactis for intracellular expression of peanut allergen and application thereof
A technology of Lactococcus lactis and peanut allergens, applied in the field of bioengineering, can solve the problems of high cost of purification of expression products, limitation of clinical application of E. coli prokaryotic expression system, complex structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Codon Optimization of Arah2 Gene Sequence
[0019] Firstly, the signal peptide sequence of Arah2's amino acid sequence (genbank: AAN77576.1) was predicted using the signal peptide prediction software SignalP 4.1 Server, and the predicted sequence was removed from the gene sequence (genbank: AY158467.1) corresponding to the above amino acid sequence to obtain The signal peptide sequence to obtain the original nucleotide sequence to be optimized.
[0020] The original nucleotide sequence was optimized according to the preferred codon table of Lactococcus lactis, and the optimized gene was named nArah2, which was synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. and subcloned into the vector pUC57 to obtain pUC57 -nArah2 (synthesized by Shanghai Sangon, where pUC57 is a commercial plasmid).
[0021] The natural gene sequence before optimization (genbank: AY158467.1), such as SEQ ID No.1.
[0022] Optimized gene sequence (nArah2), SEQ ID No.2.
[0023] ...
Embodiment 2
[0025] Example 2 Construction of recombinant expression vector pNZ1
[0026] Using the plasmid pUC57-nArah2 (synthesized by Shanghai Sangon) as a template, the nArah2 gene was amplified by PCR using primers Pra1F 5'-CATGCCATGGGTCAACAATGGGAATTACAAG-3' and Pra1R: 5'-CGGGGTACCTTAATAACGATCACGACCAC-3'. The PCR reaction system (total volume: 50 μL) is: 10×KOD plus buffer: 5 μL; dNTP: 5 μL; MgSO4: 2 μL; DNA template: 2 μL; upstream primer / downstream primer (20 μM): each 1 μL; KOD plus: 0.5 μL; wxya 2 O: 33.5 μL. Reaction program: 95°C for 30s (denaturation), 61°C for 30s (annealing), 68°C for 50s (extension), 30 cycles of amplification. The purified nArah2 gene fragment was detected and recovered by 1.0% agarose gel electrophoresis. The recovered and purified amplified fragment and vector pNZ8148 (Mierau and Kleerebezem.10years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis.Appl Microbiol Biotechnol.2005,68(6):705-717) were digested with double enzymes...
Embodiment 3
[0027] Example 3 Preparation of recombinant Lactococcus lactis
[0028]The above constructed plasmid pNZ1 was electroporated to transform L. lactis NZ9000 (Kuipers et al. Quorum sensing-controlled gene expression in lactic acid bacteria. Journal of Biotechnology. 1998, 64(1): 15-21) competent cells. Pick a single colony for colony PCR and double enzyme digestion verification and send it to Sangon Bioengineering (Shanghai) Co., Ltd. for sequencing verification. The sequenced correct transformant will be named L.lactis NZNH, and the empty plasmid strain L.lactis NZ9000 will be carried / pNZ8148 designated L. lactis NZ48.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com