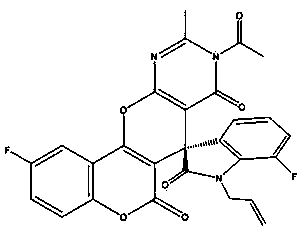
Application of chiral spiro indole-pyran pyrimidine base compound in preparation of anti-inflammatory drugs
A technology of pyrimidine bases and anti-inflammatory drugs, applied in the field of biochemistry, can solve problems such as differences in pharmacodynamics and metabolic kinetics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, the preparation of tablet
[0033] Formula: JP-8g (purity greater than 95%) 20.0g
[0034] Filler 180.0g
[0035] Disintegrant 10.0g
[0036] Adhesive 6.0g
[0037] Lubricant 3.0g
[0038] Total 200.0g
[0039] Process: 1000 tablets were made according to the conventional process for preparing tablets, each containing 20 mg of JP-8g.
[0040] Usage and dosage: twice a day, 2~3 tablets each time.
Embodiment 2
[0041] Embodiment 2: the preparation of soft capsule
[0042] Formula: JP-8g (purity greater than 95%) 50.0g
[0043] Filler 85.0g
[0044] Adhesive 5.0g
[0045] Lubricant 10.0g
[0046] Total 200.0g
[0047] Process: According to the conventional process for preparing capsules, 1000 capsules are made, and each capsule contains 50 mg of JP-8g.
[0048] Usage and dosage: twice a day, 2~3 capsules each time.
Embodiment 3
[0049] Embodiment 3, the preparation of injection
[0050] Formula: JP-8g (purity greater than 95%) 100.0g
[0051] Citric acid 1.0g
[0052] Sodium citrate 0.5g
[0054] Water for injection 2000ml
[0055] Process: According to the conventional process for preparing injections, a total of 1000 2ml injections were prepared, each containing 100 mg of JP-8g.
[0056] Usage and dosage: twice a day, 1~2 sticks each time.
[0057] The fillers, disintegrants, binders, lubricants and other auxiliary materials in the above-mentioned embodiments are the most conventional auxiliary materials in pharmacy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com