A new proline-specific endoprotease gene and its application
An endoprotease and specific technology, applied in the application, genetic engineering, plant gene improvement and other directions, can solve the problems of the proline specific endoprotease coding sequence and preparation method, and improve the non-biological stability of beer. performance, increased yield, good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0046] Example 1A. Acquisition of oryzae proline-specific endoprotease gene
[0047] Based on Aeromonas (Genbank: AF065429), Pseudomonas capsularis (Genbank: AB010298), Myxococcus aureus (Genbank: ABF89794) and Aspergillus fumigatus (Genbank: XM744168) and Aspergillus niger (Genbank: AX458699) Degenerate primers were designed for the proline-specific endoprotease protein sequence as follows:
[0048] g1: 5-RASMTWSRYATYASGGRA-3
[0049] g2: 5-SVBYCBCYMBRGRKMANRNTB-3
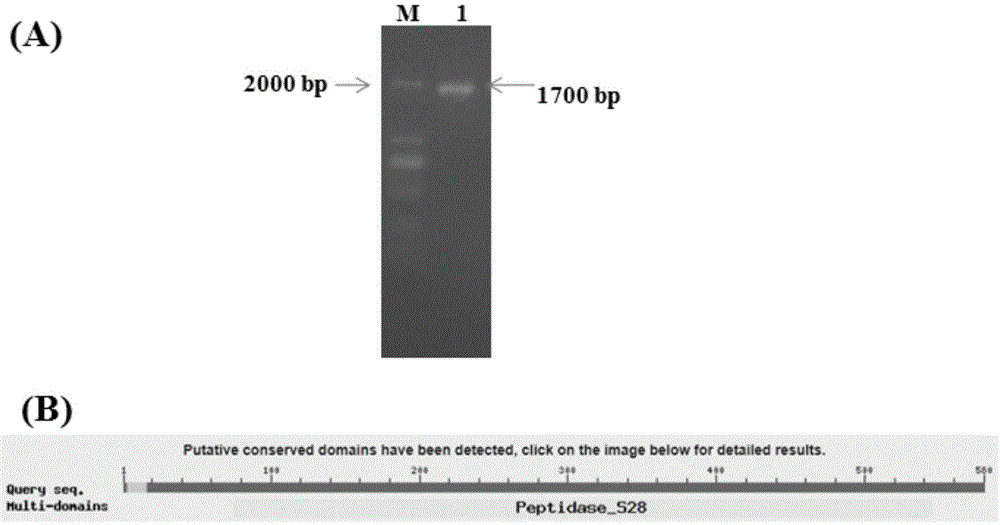
[0050] Using the Aspergillus oryzae cDNA as the template and g1 and g2 as the primers for PCR, a 1000bp product fragment was obtained. After the product was sequenced and compared at NCBI, it was found that it was all related to the A.oryzae protease gene (SequenceID: XM_001825944.2) The conserved sequence homology is high.
[0051] Furthermore, we redesigned the primers according to the sequence of the A.oryzae protease gene (SequenceID: XM_001825944.2) published in the GenBank database, and carried out PCR. T...
Embodiment 2
[0072] Example 2 Obtaining the predicted crystal structure of A.oryzae proline-specific endoprotease by "homology modeling" method and its amino acid similarity with other Aspergillus proline-specific endoprotease
[0073] The full length of the Aspergillus oryzae proline-specific endoprotease gene S2 is 1743bp, and the predicted open reading frame of the new protein is located at nucleotides 64-1743, encoding 558 amino acid residues, with a molecular weight of 65kDa.
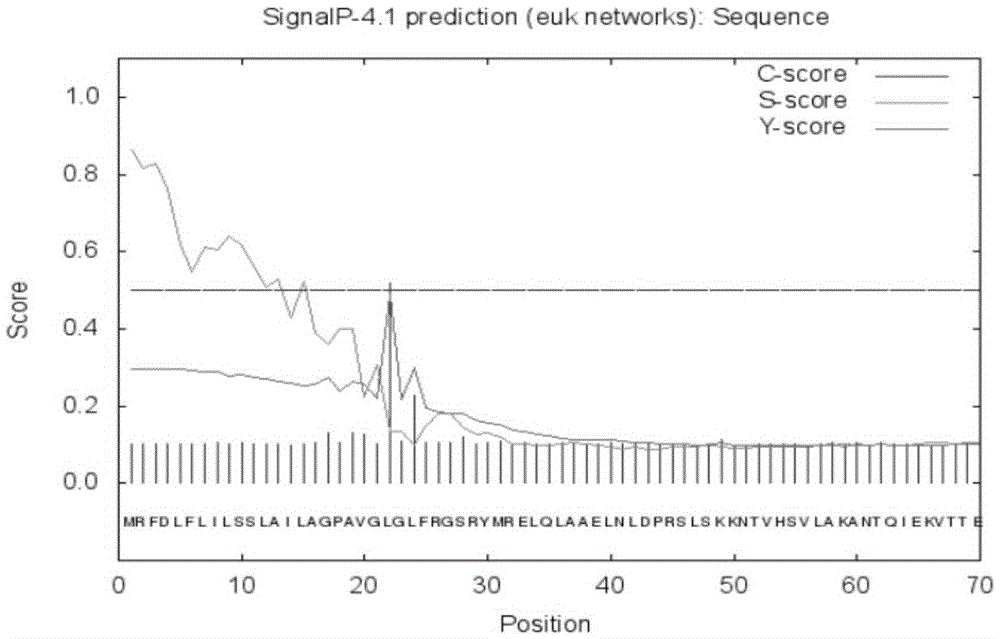
[0074] According to SignalP prediction, the probability of the N-terminal of the protein being a signal peptide is 86.4%, and the signal peptide cleavage site is located between amino acids 21 and 22 (see figure 2 ).
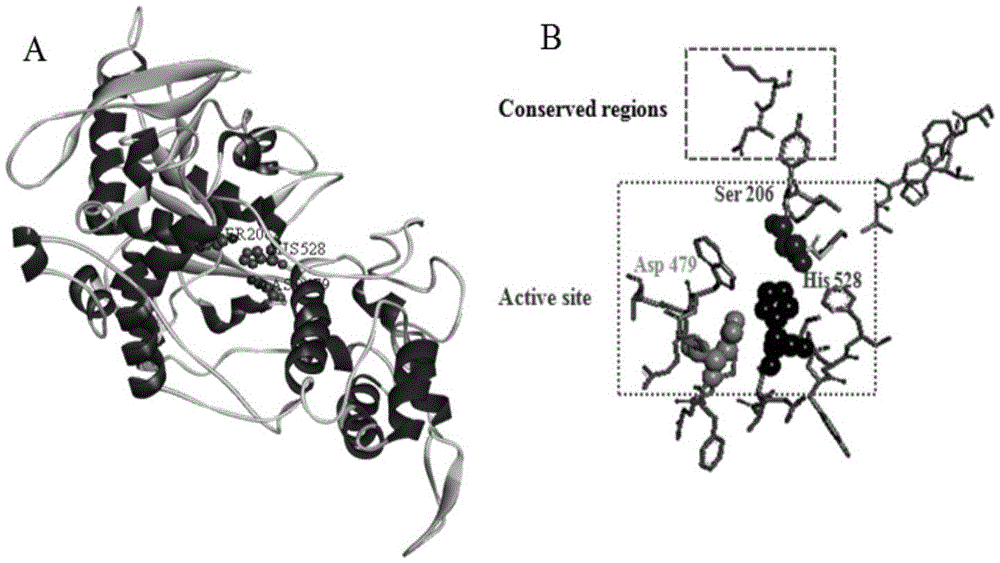
[0075]Submit the amino acid sequence of A. oryzae proline-specific endoprotease to the SWISS-MODEL protein online modeling server (http: / / swissmodel.expasy.org / ) for homology modeling, and then use Discoverystudio software to analyze Aspergillus oryzae Amino acid-specific endoprotease protein h...
Embodiment 3
[0077] Example 3 Construction of Aspergillus oryzae proline-specific endoprotease eukaryotic expression vector, recombinant expression and protein expression thereof
[0078] 1. Construction of eukaryotic expression vector
[0079] 1) Primer design: design primers starting from the mature peptide sequence after the signal peptide
[0080] g5:5′-CGG TACGTA TTGGGGTTGTTTAGAGG-3';
[0081] g6:5'-CC GCGGCCGC CTACATCACCGCCCCCTTTG-3';
[0082] 2) PCR reaction, using the cloning vector pMD-19T-S2 as a template, annealing at 62°C, 35 cycles.
[0083] 3) SnaBI and NotI double digestion PCR product of S2 and plasmid pPIC-9
[0084] Element
Usage amount
Purification of PCR products / plasmids
30μl
10*quitcut buffer
5μl
QuitCut SnaBI
1μl
QuitCut NotI
1μl
ddH 2 O
13μl
total capacity
50μl
[0085] Digest at 37℃ for 2hr
[0086] 4) Ligate, transform, and identify by double enzyme digestion according...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com