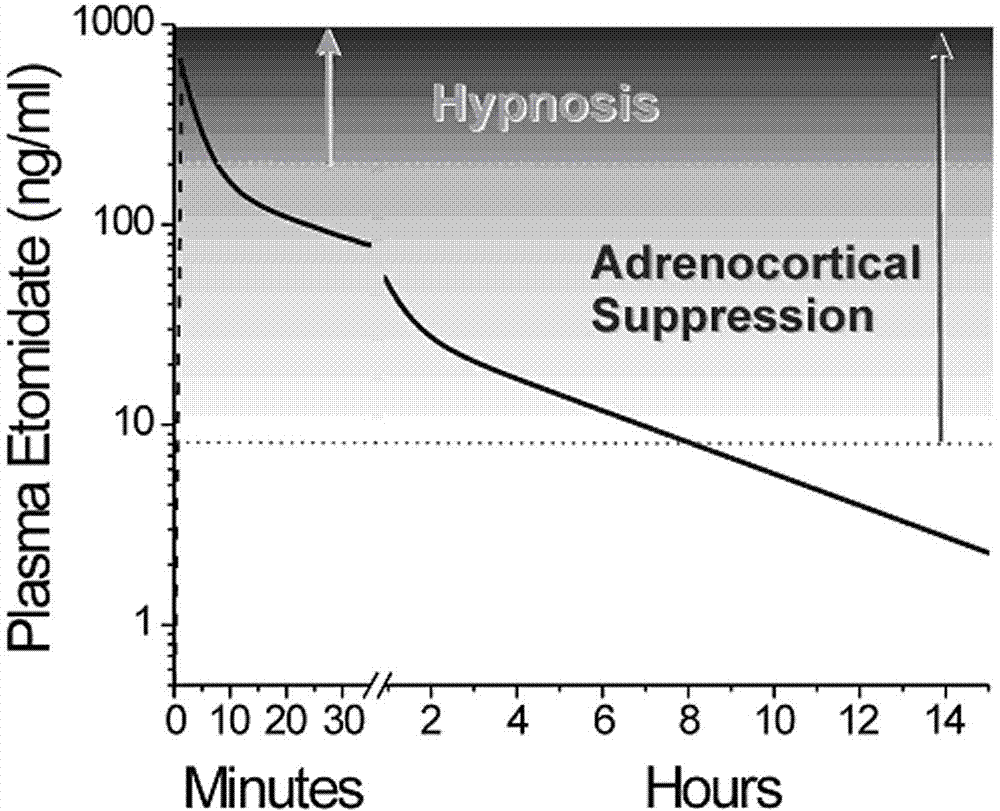
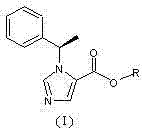
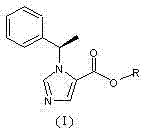
N-substituted imidazole carboxylic ester compound with ultrashort-acting anesthetic effect, preparation method and use thereof
A technology of ester compounds and imidazole carboxylic acid, which is applied in the field of N-substituted imidazole carboxylic acid ester compounds, can solve the problems of imidazole derivatives with weak 11-β hydroxylase binding ability, strengthen drug molecules and enzyme molecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]N-substituted imidazole carboxylic acid compound (II) (2g, 9.26mmol) and ethylene glycol monoacetate (III) (0.96g, 9.26mmol) were dissolved in 50ml of dichloromethane, and a catalytic amount of DMAP (4- Dimethylaminopyridine), add dropwise 30ml of dichloromethane solution containing DCC (N,N-dicyclohexylcarbodiimide, 1.92g, 9.30mmol), stir at room temperature for 2 hours after dropping, and use thin layer chromatography Spot plate observation, N-substituted imidazole carboxylic acid compound (II) reacted completely. Filtrate, concentrate the filtrate under reduced pressure, add a small amount of ethyl acetate to dissolve the residue, put it in a refrigerator at minus 20°C overnight, filter out a small amount of precipitated solid the next day, and concentrate the filtrate to obtain a light yellow oil, which is subjected to silica gel column chromatography (developed Reagent: cyclohexane / ethyl acetate=7 / 1) to obtain 1.82 g of colorless and transparent oily product (I) (n=...
Embodiment 2
[0042] Dissolve N-substituted imidazole carboxylic acid compound (II) (2g, 9.26mmol) and ethylene glycol monoacetate (III) (0.96g, 9.26mmol) in 50ml DMF, add a catalytic amount of DMAP, dropwise add DCC ( 1.92g, 9.30mmol) in 30ml of DMF solution, stirred at room temperature for 2 hours after dropping, spot plate observation, N-substituted imidazole carboxylic acid compound (II) reacted completely. Pour the reaction solution into 300ml of water, add 200ml of ethyl acetate for extraction, separate the organic layer, wash once with water, add anhydrous sodium sulfate to the organic layer and dry overnight, filter the desiccant out the next day, concentrate the filtrate by evaporation under reduced pressure, add a small amount of acetic acid Dissolve the residue in ethyl ester, put it in minus 20°C refrigerator overnight, filter out a small amount of precipitated solid the next day, and concentrate the filtrate to obtain a light yellow oily product (I) (n=2, R' is acetyl group), wh...
Embodiment 3
[0044] Dissolve N-substituted imidazole carboxylic acid compound (II) (2g, 9.26mmol) and bromoethanol (IV) (1.16g, 9.26mmol) in 50ml DMF, add powdered sodium hydroxide (0.37g, 9.26mmol), in Stir at 60°C for 1 hour, spot plate observation, the reaction of N-substituted imidazole carboxylic acid compound (II) is complete. Pour the reaction solution into 250ml of water, add 150ml of ethyl acetate for extraction, separate the organic layer, wash once with water, add anhydrous sodium sulfate to the organic layer and dry overnight, filter the desiccant the next day, and evaporate the filtrate under reduced pressure to concentrate, and the crude product obtained is directly for the next reaction.
[0045] Dissolve the product obtained in the previous step in 50ml of DMF, add acetic acid (Ⅴ) (0.56g, 9.26mmol), add a catalytic amount of DMAP, then add 30ml of DMF solution containing DCC (1.92g, 9.30mmol) dropwise, and stir at room temperature after dropping 2 hours. Pour the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com