Method for preparing 2-methoxy-4-amino-5-ethysulfonyl benzoic acid methyl ester by halogen halogenation
A technology of methyl ethylsulfonyl benzoate and methyl acetamidobenzoate, applied in the field of pharmaceutical intermediate preparation, can solve problems such as difficult removal, low yield, poor quality, etc., and achieve good product quality and high product yield , the effect of simple process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
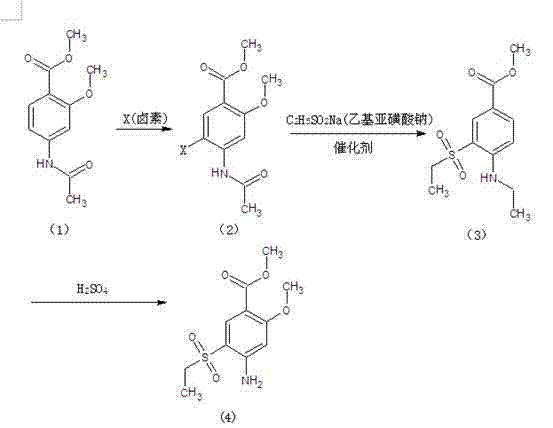
[0018] Add 300g of dichloromethane and (1) 200g (0.89mol) of methyl 2-methoxy-4-acetamidobenzoate into a reaction flask equipped with a hydrogen chloride absorption device, cool to below 10°C, and stir Maintain 10-15°C and slowly feed chlorine gas, when the gas flow reaches 69.5g (0.98mol), stop the chlorine gas flow, continue to stir and react for 1 hour, then concentrate the reaction solution under negative pressure to dryness to obtain solid (2) 2-methoxy Base-4-acetamido-5-chlorobenzoic acid methyl ester, add 400 grams of DMF, 155 g (1.33 mol) of sodium ethyl sulfinate, 6 g (0.04 mol) of cuprous oxide in the same reaction flask, after the addition is completed, slowly Raise the temperature to 65-70°C and keep it at this temperature for 8 hours. After the heat preservation is over, cool it down to below 10°C and filter to get (3) methyl 2-methoxy-4-acetamido-5-ethylsulfonylbenzoate , add (3) into the reaction flask with a stirring reflux device, add 300ml of methanol and 5 ...
Embodiment approach 2
[0020] Add 225g of dichloromethane and (1) 150g (0.67mol) of methyl 2-methoxy-4-acetamidobenzoate into a reaction flask equipped with a hydrogen bromide absorption device, cool to below 10°C, and stir Slowly add bromine 112.4g (0.70mol) dropwise at 10-15°C, keep the temperature at 10-15°C for 4 hours at the end of the dropwise addition, and concentrate the reaction solution under negative pressure to dryness to obtain solid (2) 2-methoxy -4-Acetamido-5-bromobenzoic acid methyl ester, add 300 grams of DMF, 124.5g (1.07mol) sodium ethyl sulfinate, 5g (0.05mol) cuprous chloride in the same reaction flask, the addition is complete, Slowly raise the temperature to 75-80°C, and keep it warm at this temperature for 8 hours. After the heat preservation is over, cool it down to below 10°C, and filter to get (3) 2-methoxy-4-acetamido-5-ethylsulfonylbenzoic acid methyl ester, add (3) into a reaction flask with a stirring reflux device, add 250ml of methanol and 4 grams of concentrated su...
Embodiment approach 3
[0022] Add 600g of dichloromethane, (1) 300g (1.34mol) of methyl 2-methoxy-4-acetamidobenzoate, and 390g (1.54mol) of iodine into a 1000ml reaction bottle. After the addition is complete, heat up to 35-40°C , and kept at this temperature for 10 hours, after the end of the heat preservation, the reaction solution was concentrated to dryness under negative pressure to obtain a solid (2) 2-methoxy-4-acetamido-5-chlorobenzoic acid methyl ester, added 600 grams of DMF, 417g (2.68mol) of sodium ethyl sulfinate and 8g (0.04mol) of cuprous iodide, after feeding, slowly raise the temperature to 65-70°C, and keep it at this temperature for 10 hours, after the end of the heat preservation, cool To below 10°C, filter to obtain (3) methyl 2-methoxy-4-acetamido-5-ethylsulfonylbenzoate, add (3) to a reaction flask with a stirring reflux device, and add 450ml of methanol And 8 grams of concentrated sulfuric acid, heat up to reflux and keep warm for 5 hours under reflux conditions. After the h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com