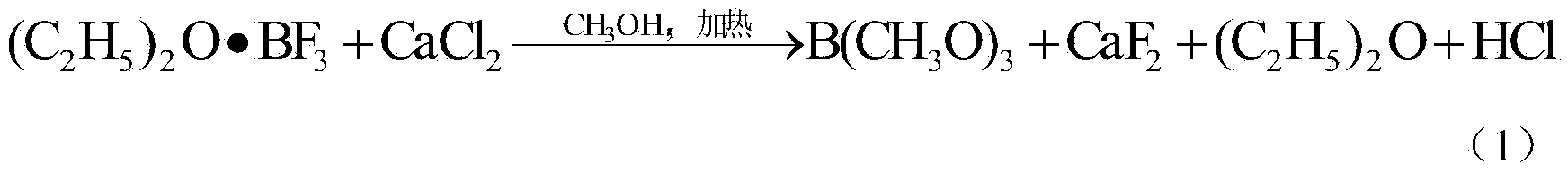Preparation method of boron-10 acid
A technology of lithium carbonate and boron trifluoride, which is applied in the direction of boron oxide compounds, can solve the problems of high consumption of lithium chloride, low reaction yield, hydrolysis of trimethyl borate, etc., and achieve high yield and purity of boric acid. The effect of simple process and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh powdered lithium carbonate (Li 2 CO 3 ) 55.4g (0.75mol, the ratio of boron trifluoride to lithium carbonate is 1:1.5), add 277.0g water to dissolve, forming a solid-liquid mixture of lithium carbonate and water. After heating the solid-liquid mixture to 40°C, slowly introduce enriched boron trifluoride at a rate of 10ml / min 10 BF 3 33.9 g (0.5 mol), while stirring constantly, boron trifluoride 10 BF 3 The access time is about 2h. After the completion of the boron trifluoride gas introduction, continue to maintain the water bath at 40°C for 25 hours to promote the occurrence of reaction (6).
[0029] After the reaction is completed, the pH of the solution is around 7, and the generated lithium fluoride is a solid precipitate that is very easy to filter. Filtrate at room temperature to separate the lithium fluoride from the mother liquor, and wash the solid filter cake with about 100 g of deionized water, and mix the obtained washing liquor with the mother liq...
Embodiment 2
[0033] Weigh powdered lithium carbonate (Li 2 CO 3 ) 57.3g (0.775mol, the ratio of boron trifluoride to lithium carbonate is 1:1.55), dissolved in 315.2g of water to form a solid-liquid mixture of lithium carbonate and water. After heating the solid-liquid mixture to 60°C, slowly add enriched boron trifluoride at a rate of 25ml / min 10 BF 3 33.9 g (0.5 mol), while stirring constantly, boron trifluoride 10 BF 3 The access time is about 0.8h. After the boron trifluoride gas is introduced, continue to react in a water bath at 60°C for 15 hours to promote the occurrence of reaction (6).
[0034] After the reaction is completed, the pH of the solution is around 7, and the generated lithium fluoride is a solid precipitate that is very easy to filter. Filtrate at room temperature to separate the lithium fluoride from the mother liquor, and wash the solid filter cake with about 100 g of deionized water, and mix the obtained washing liquor with the mother liquor.
[0035] All the...
Embodiment 3
[0038] Weigh powdered lithium carbonate (Li 2 CO 3 ) 59.1g (0.8mol, the ratio of boron trifluoride to lithium carbonate is 1:1.6), add 354.6g of water to dissolve, forming a solid-liquid mixture of lithium carbonate and water. After heating the solid-liquid mixture to 80°C, slowly add enriched boron trifluoride at a rate of 40ml / min 10 BF 3 33.9 g (0.5 mol), while stirring constantly, boron trifluoride 10 BF 3 The access time is about 0.5h. After the completion of the boron trifluoride gas introduction, continue to maintain the water bath at 80°C for 10 hours to promote the occurrence of reaction (6).
[0039] After the reaction is completed, the pH of the solution is around 7, and the generated lithium fluoride is a solid precipitate that is very easy to filter. Filtrate at room temperature to separate the lithium fluoride from the mother liquor, and wash the solid filter cake with about 100 g of deionized water, and mix the obtained washing liquor with the mother liquo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com