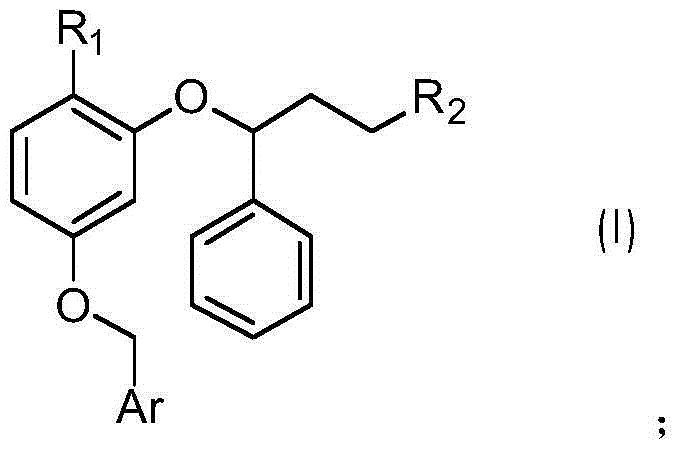
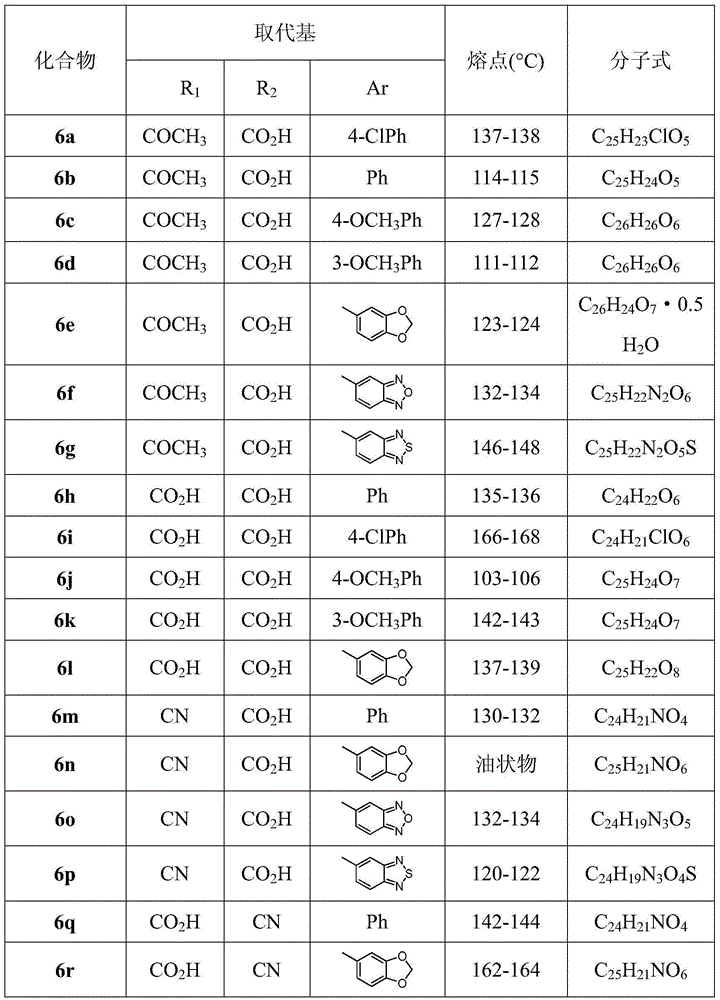
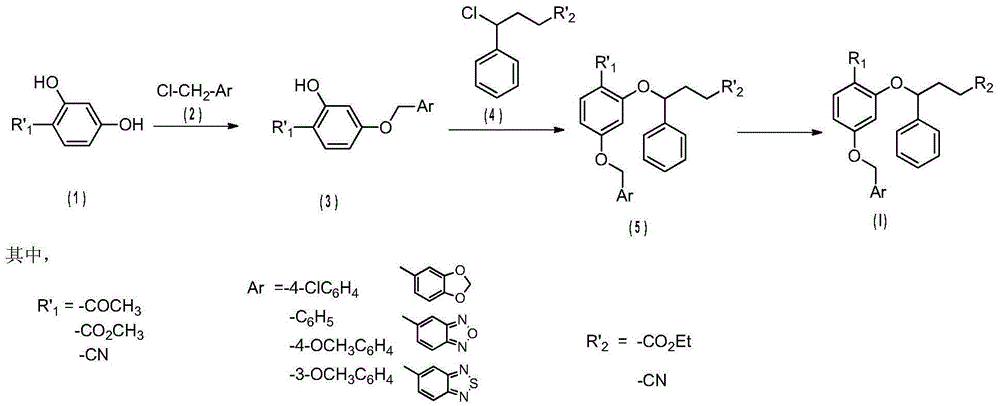
2,4-dibenzyloxybenzoic acid derivatives and their preparation methods and applications
A technology of dibenzyloxybenzoic acid and derivatives, applied in 2 fields, can solve the problems of low oral bioavailability of peptide antagonists, limit the application of cardiovascular diseases, etc., and achieve the effect of strong endothelin receptor antagonistic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0051] Embodiment 12, the preparation of 4-dihydroxybenzonitrile (1c)
[0052] (1) Preparation of 2,4-dihydroxybenzaldehyde oxime (1c1)
[0053] Dissolve 28g (0.4mol) of hydroxylamine hydrochloride in 20ml of water, drop into 20ml of aqueous solution of 23g (0.41mol) of KOH under cooling, add 50ml of absolute ethanol and 20ml of glacial acetic acid to prepare this solution for later use. Dissolve 50g (0.362mol) of 2,4-dihydroxybenzaldehyde in 150ml of absolute ethanol, add the above standby solution under cooling, after addition, reflux for 0.5hr, cool to room temperature, filter the resulting potassium chloride, and concentrate the filtrate To dryness, the residue was recrystallized from water to obtain 53.5 g of white needle crystals, yield 96%, mp 191-192°C (191°C in literature).
[0054] (2) Preparation of 2,4-diacetoxybenzonitrile (1c2)
[0055] React 3.4g (0.022mol) of compound 2,4-dihydroxybenzaldehyde oxime with 20ml of acetic anhydride under reflux for 8 hours, cool...
Embodiment 2
[0057] Saponify 60g (0.274mol) of the compound 2,4-diacetoxybenzonitrile in a 700ml aqueous solution containing 50g NaOH for 1.5hr at room temperature, then adjust the pH to 3 with 10% HCl, extract the aqueous solution with 5×100ml ether, Dry over anhydrous magnesium sulfate, distill off the solvent to obtain 34 g of white needle crystals, yield 92%, mp 179-181°C (179°C in literature). The preparation of embodiment 2 p-methoxybenzyl chloride (2c)
[0058] Dissolve 27g (0.2mol) anisaldehyde in 80ml methanol, add 20ml water, add 5g (0.1mol) KBH in batches 4 , After the addition, react at room temperature for 2hr, adjust the pH=3 with 10% HCl under cooling, and then use saturated NaHCO 3 Neutralize to pH=8, evaporate methanol under reduced pressure, a large amount of oil appears, extract with toluene, wash with water and saturated NaCl successively, dry over anhydrous magnesium sulfate, evaporate the solvent to obtain 30.7 g of colorless transparent liquid, yield 98 %.
[0059...
Embodiment 35
[0061] The preparation of embodiment 35-chloromethyl-2,1,3-benzoxadiazole (2f)
[0062] (1) Preparation of 4-chloro-3-nitrobenzaldehyde (2f1)
[0063] Add 70g (0.5mol) p-chlorobenzaldehyde in batches to 55ml of fuming nitric acid and 55ml of concentrated sulfuric acid at <10°C, then react at this temperature for 2hr, after the reaction is complete, pour the reaction solution into ice water, The precipitated oil was solidified, filtered, and recrystallized with 70% ethanol to obtain 90 g of white needle crystals, yield 97%, mp62-63°C.
[0064] (2) Preparation of 4-azido-3-nitrobenzaldehyde (2f2)
[0065] Dissolve 46.4g (0.25mol) of the compound 4-chloro-3-nitrobenzaldehyde in 300mlDMSO, and add 18g (0.276mol) of NaN 3 , control the temperature <30°C, then stir at room temperature for 1 hr, after the reaction is complete, pour the reaction solution into 100ml of ice water to precipitate a light yellow powdery solid, filter, wash with water until the filtrate is colorless, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com