Benzofuran endothelin receptor antagonist and use
An endothelin receptor and benzofuran technology, which is applied in the field of medicine and chemical industry, can solve the problems of limiting the application of cardiovascular diseases, low oral bioavailability of peptide antagonists, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
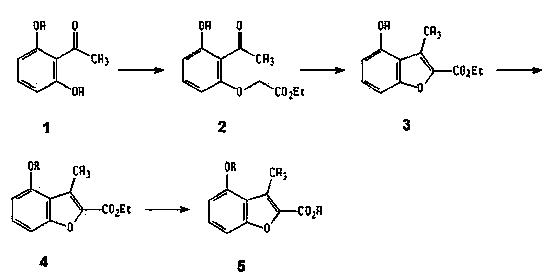
[0020] Example 1 Ethyl 1-(2-acetyl-3-hydroxy)phenoxyacetate
[0021] With 15.2g (0.1mol) 2,6-dihydroxyacetophenone, 41.4g (0.3mol) K 2 CO 3 Mix in 200ml of acetone, slowly drop in 20g (0.12mol) of ethyl bromoacetate, reflux for 3 hours after dropping, cool to room temperature, filter, wash the filter cake with 20ml of acetone, after the filtrate is concentrated, add an appropriate amount of water, and white Flaky crystals were filtered, the product was washed with 50% acetone-water solution, and dried to obtain 14.6 g of the product, yield 61%, mp80-82°C.
Embodiment 2
[0022] Example 2 1-Methyl-4-hydroxybenzofuran-2-carboxylic acid ethyl ester
[0023] Add 13.6g (0.057mol) ethyl 1-(2-acetyl-3-hydroxy)phenoxyacetate to a sodium ethoxide solution made of 2.7g (0.118mol) sodium metal and 180ml absolute ethanol, then Heating to reflux for 9 hours, cooling to room temperature, pouring the reaction solution into a large amount of ice water, adjusting the pH to 3 with concentrated HCl, granular solids precipitated, and suction filtering after standing, the filter cake was washed with water, dried, and the product was washed with a flash column Chromatography (eluent: petroleum ether: ethyl acetate = 2:1 (v / v)) purified to give 4.5 g of white powdery solid, yield 36%, mp 158-159 °C. 1 H-NMR (CDCl 3 )δ1.44 (t, 3H, -OCH 2 C H 3 ), 2.78 (s, 3H, 3-CH 3 ), 4.45 (q, 2H, -OC H 2 CH 3 ), 6.6 (d, 1H, 5-H), 7.12 (d, 1H, J=4Hz, 7-H), 7.26 (t, 1H, J=4Hz, 6-H).
Embodiment 3
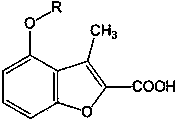
[0024] Example 3 4-(3-phenylallyloxy)-3-methylbenzofuran-2-carboxylic acid (Ⅰ 1 )
[0025] Dissolve 0.9g (3.6mmol) ethyl 1-methyl-4-hydroxybenzofuran-2-carboxylate in 20ml DMF, add 3.5g K 2 CO 3 , 0.5g KI, add 1ml of phenyl allyl bromide under mechanical stirring, then heat to 60°C, the reaction solution changes from yellow-green fluorescence to colorless, then stir for 0.5hr, cool to room temperature, pour the reaction solution into ice In water, the precipitated oil was solidified and then recrystallized from methanol to obtain 0.76 g of colorless granular crystal 4-(3-phenylallyloxy)-3-methylbenzofuran-2-carboxylic acid ethyl ester.
[0026] Dissolve 0.7g (3mmol) ethyl 4-(3-phenylallyloxy)-3-methylbenzofuran-2-carboxylate in 30ml ethanol, add 10ml NaOH, reflux for 1hr, cool to room temperature , adjusted pH=3 with 10% HCl under cooling, recrystallized the precipitated solid with 75% ethanol to obtain light yellow granular crystals, yield 67%, mp191-193°C. Anal.C 19 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com