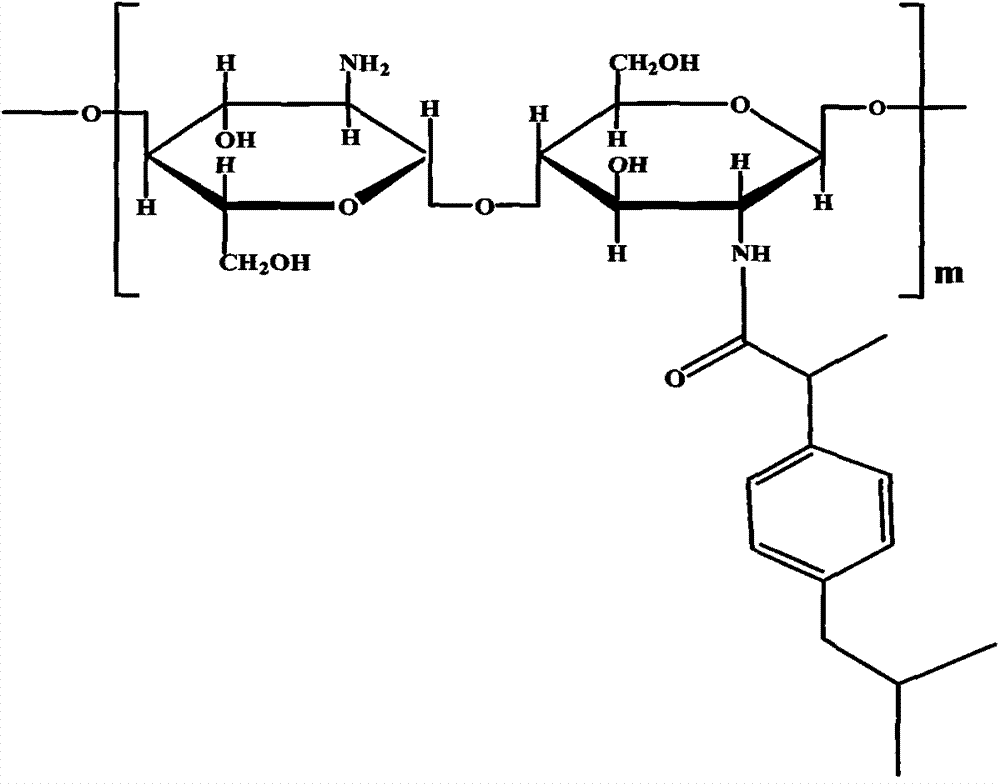
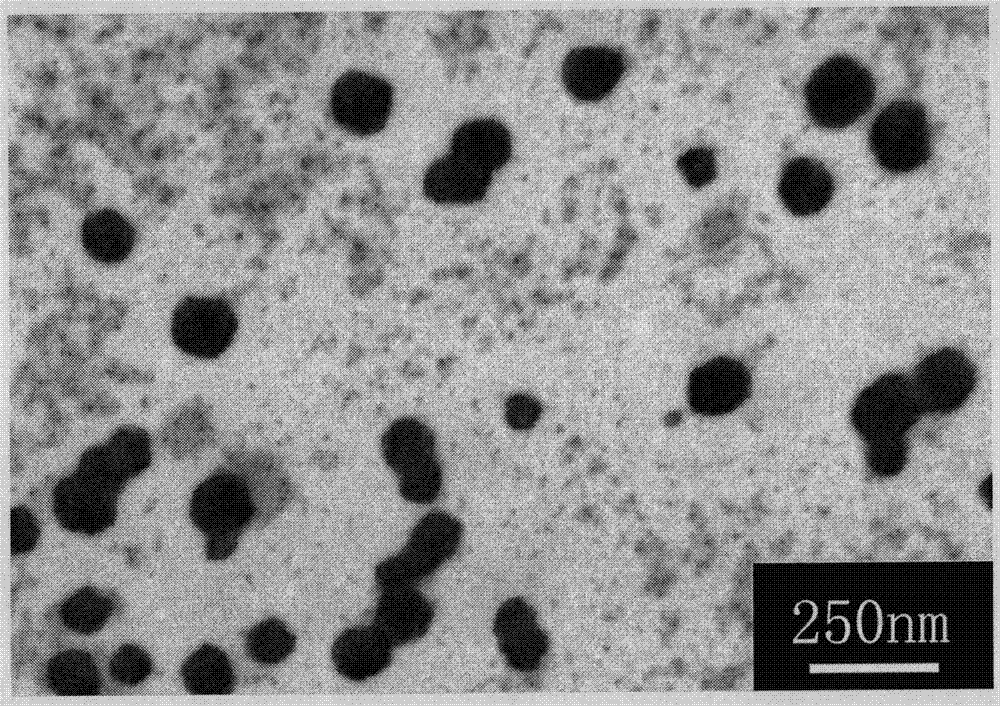
Preparation method of chitosan oligosaccharide nanoparticle
A technology of chitosan oligosaccharide and nanoparticles, which is applied in the directions of non-active ingredients medical preparations, pharmaceutical formulations, non-central analgesics, etc., can solve problems such as limiting wider application, and achieve increased drug loading and long drug release. Good effect of time and affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 0.1g chitosan oligosaccharide was dissolved in 6ml double distilled water, 0.06g ibuprofen and 0.4g EDC were dissolved in acetone. Add the acetone solution of ibuprofen and EDC to the double distilled aqueous solution of chitosan oligosaccharide under rapid stirring, and react at a constant temperature of 25° C. in a water bath for 36 hours under magnetic stirring. The resulting product was dialyzed with double distilled water for 12 hours in a semipermeable membrane, then dialyzed with absolute ethanol for 12 hours, then dialyzed with double distilled water for 24 hours, finally centrifuged and freeze-dried to obtain chitosan-ibuprofen conjugates . Mix equal concentrations of paclitaxel in acetone and oligochitosan-ibuprofen conjugate aqueous solution at a ratio of 1:10, ultrasonically shake for 40 minutes, centrifuge, and freeze-dry to obtain paclitaxel-loaded oligochitosan-ibuprofen nanoparticles .
Embodiment 2
[0037] 0.1g chitosan oligosaccharide was dissolved in 6ml double distilled water, 0.04g ibuprofen and 0.3g EDC were dissolved in absolute ethanol. Add the absolute ethanol solution of ibuprofen and EDC to the double distilled aqueous solution of chitosan oligosaccharide under rapid stirring, and react at a constant temperature of 25° C. in a water bath for 53 hours under magnetic stirring. The resulting product was dialyzed with double distilled water for 24 hours in a semipermeable membrane, then dialyzed with absolute ethanol for 24 hours, then dialyzed with double distilled water for 24 hours, finally centrifuged and freeze-dried to obtain chitosan-ibuprofen conjugates . Mix an equal concentration of curcumin ethanol solution and chitosan oligosaccharide-ibuprofen conjugate aqueous solution at a ratio of 1:2, ultrasonically vibrate for 30 minutes, centrifuge, and freeze-dry to obtain curcumin-loaded chitosan-ibuprofen nanoparticles.
Embodiment 3
[0039]0.1g chitosan oligosaccharide was dissolved in 10ml double distilled water, 0.06g ibuprofen and 0.2g EDC were dissolved in absolute ethanol. Add the absolute ethanol solution of ibuprofen and EDC to the double-distilled aqueous solution of chitosan oligosaccharide under rapid stirring, and react at a constant temperature of 30° C. in a water bath for 30 hours under magnetic stirring. The resulting product was dialyzed with double distilled water for 24 hours in a semipermeable membrane, then dialyzed with absolute ethanol for 24 hours, then dialyzed with double distilled water for 24 hours, finally centrifuged and freeze-dried to obtain chitosan-ibuprofen conjugates . Mix an equal concentration of curcumin ethanol solution and chitosan oligosaccharide-ibuprofen conjugate aqueous solution at a ratio of 1:6, ultrasonically vibrate for 20 minutes, centrifuge, and freeze-dry to obtain curcumin-loaded chitosan-ibuprofen nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com