Azelnidipine preparation with combination of two disintegrating agents and preparation method of azelnidipine preparation
A technology of azedipine and disintegrant, which is applied in the field of azedipine preparations and its preparation, can solve the problems of undisclosed dosage forms for rapid release of drugs, and does not involve dissolution curve fitting, so as to achieve the effect of saving manpower and material resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
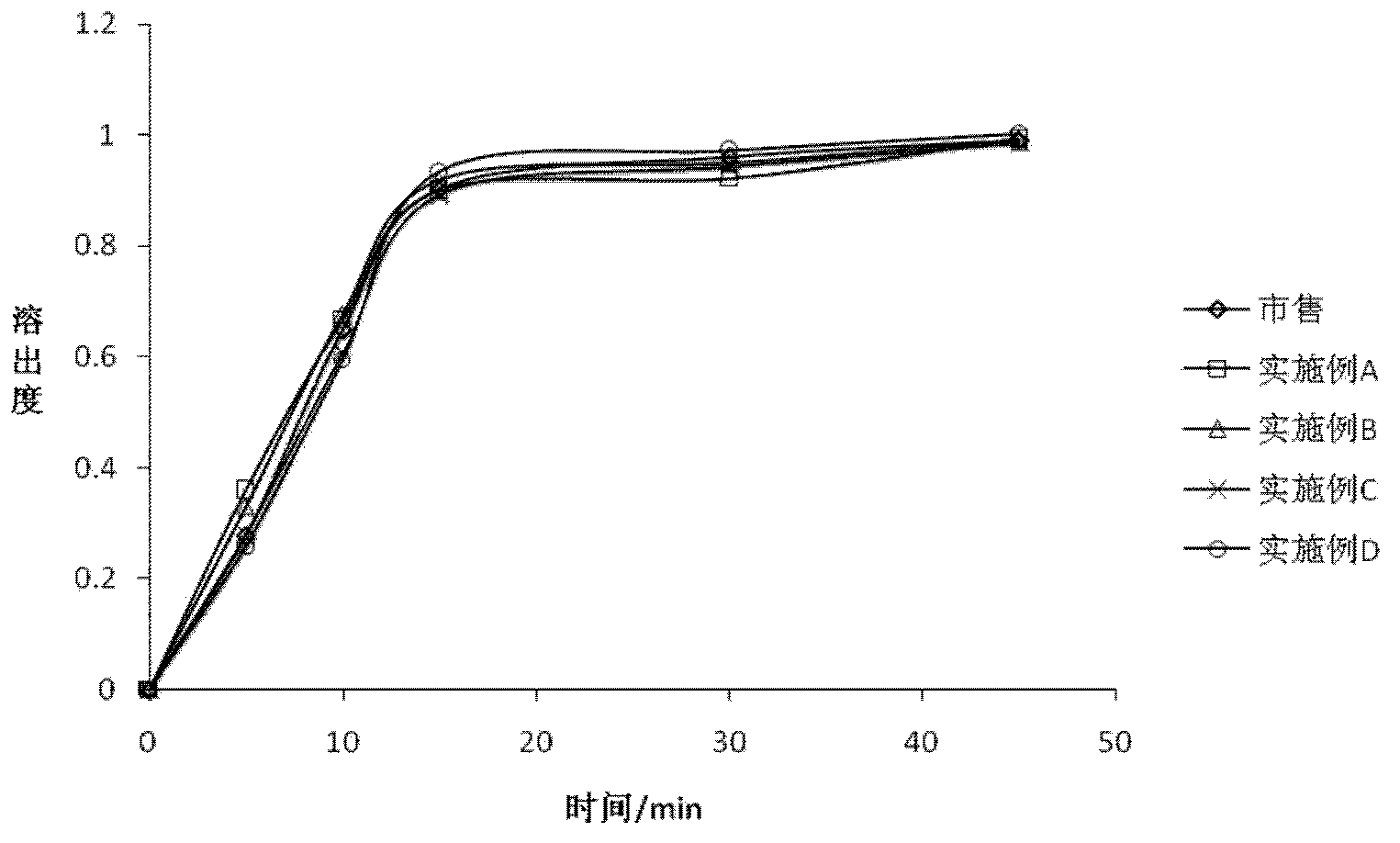
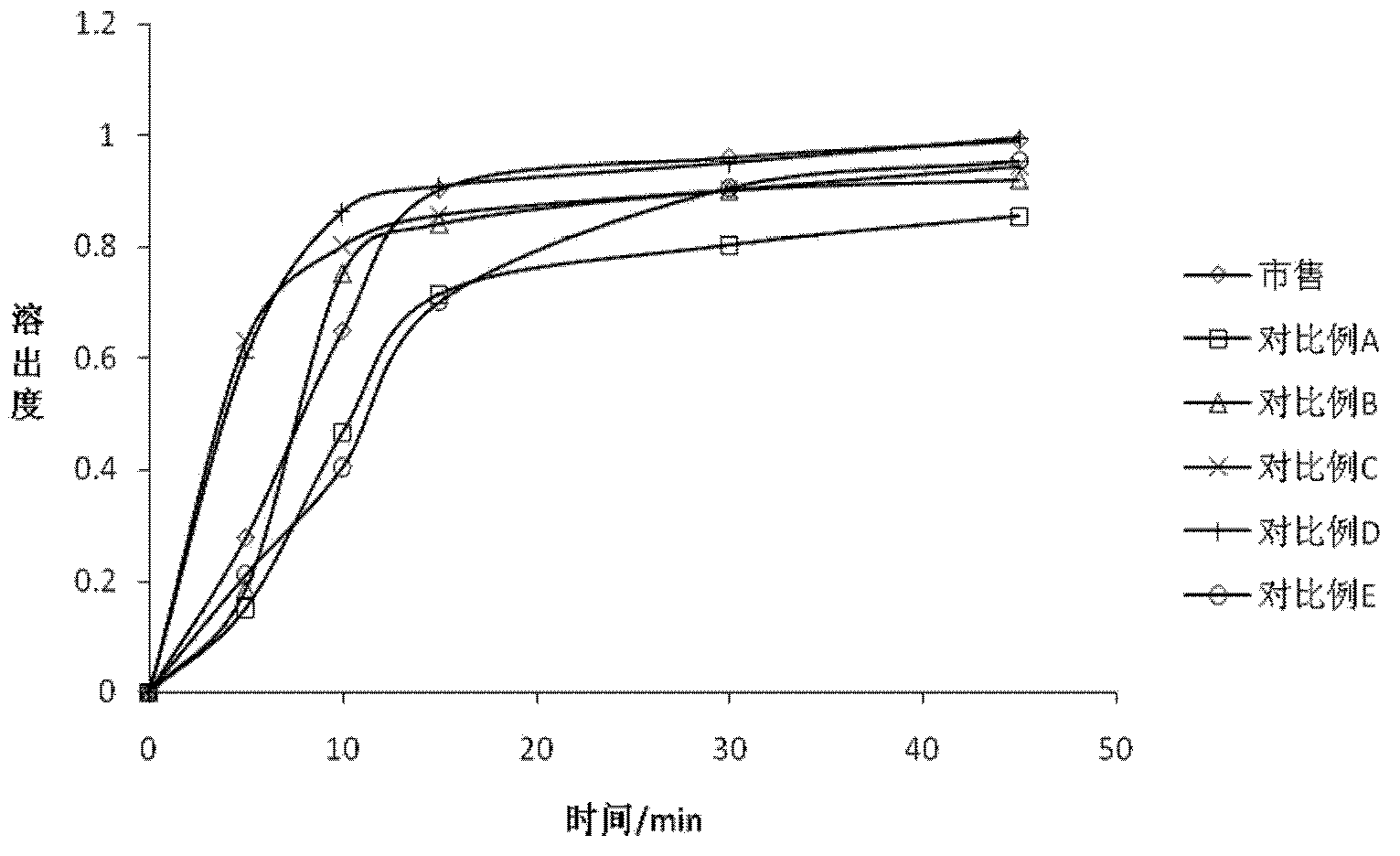
Examples
Embodiment A
[0025]
[0026] Preparation process: pass the azedipine, lactose, microcrystalline cellulose, carmellose calcium and low-substituted hydroxypropyl cellulose in the prescription through a 60-mesh sieve twice, mix well, add magnesium stearate, mix well Compressed into tablets.
Embodiment B
[0028]
[0029]
[0030] Preparation process: pass the azelnidipine, mannitol, carmellose calcium, low-substituted hydroxypropyl cellulose and micropowder silica gel in the prescription twice through a 60-mesh sieve, mix well, add magnesium stearate, mix well Pressed into tablets.
Embodiment C
[0032]
[0033] Preparation process: pass the azedipine, microcrystalline cellulose, mannitol, carmellose calcium and low-substituted hydroxypropyl cellulose in the prescription through a 60-mesh sieve twice, mix well, add magnesium stearate, mix well Compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com