Andrographolide powder preparation for injection and preparation method thereof
A technology for Yanhuning and injection, which is applied in the field of pharmaceutical preparations, can solve problems such as general medicinal effects of powder injections, increased adverse reactions of excipients, and influence on the quality of preparations, so as to avoid adverse reactions, and the freeze-drying method is scientific and reasonable, and improves the quality of preparations. The effect of resolubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
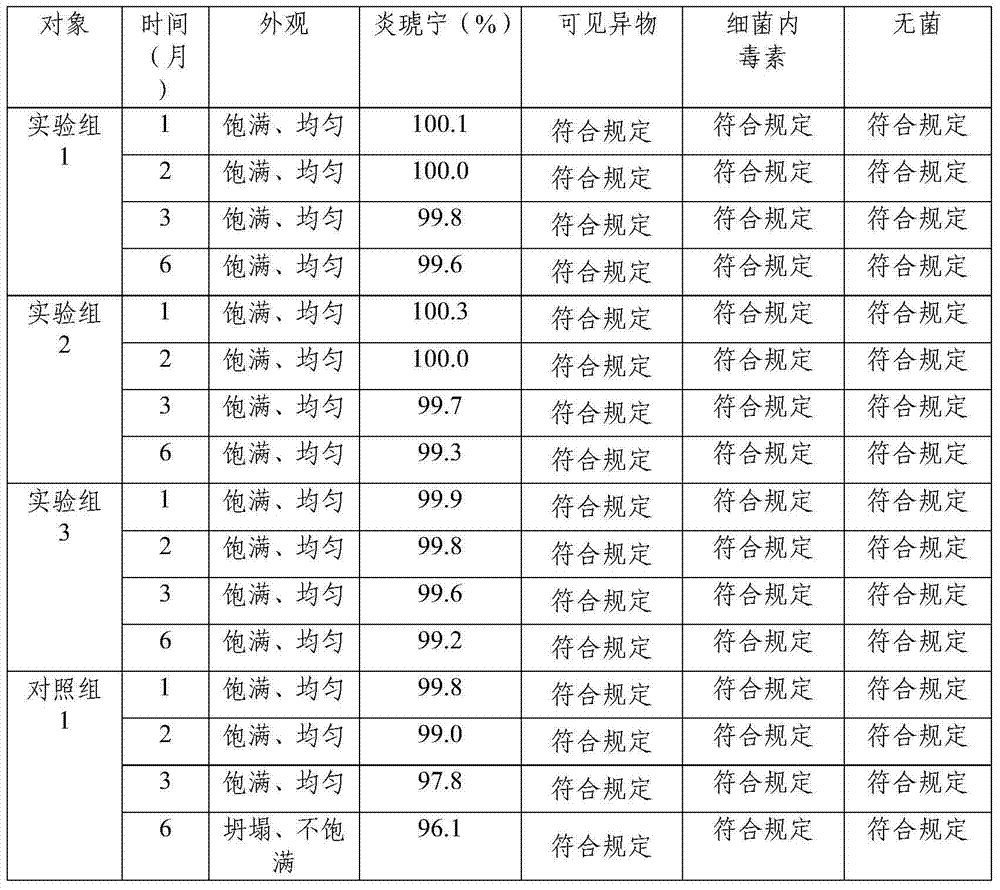
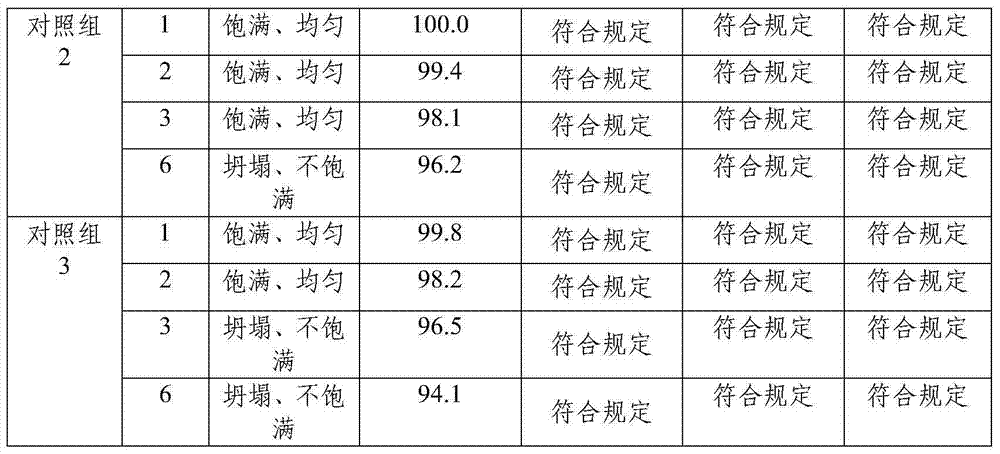
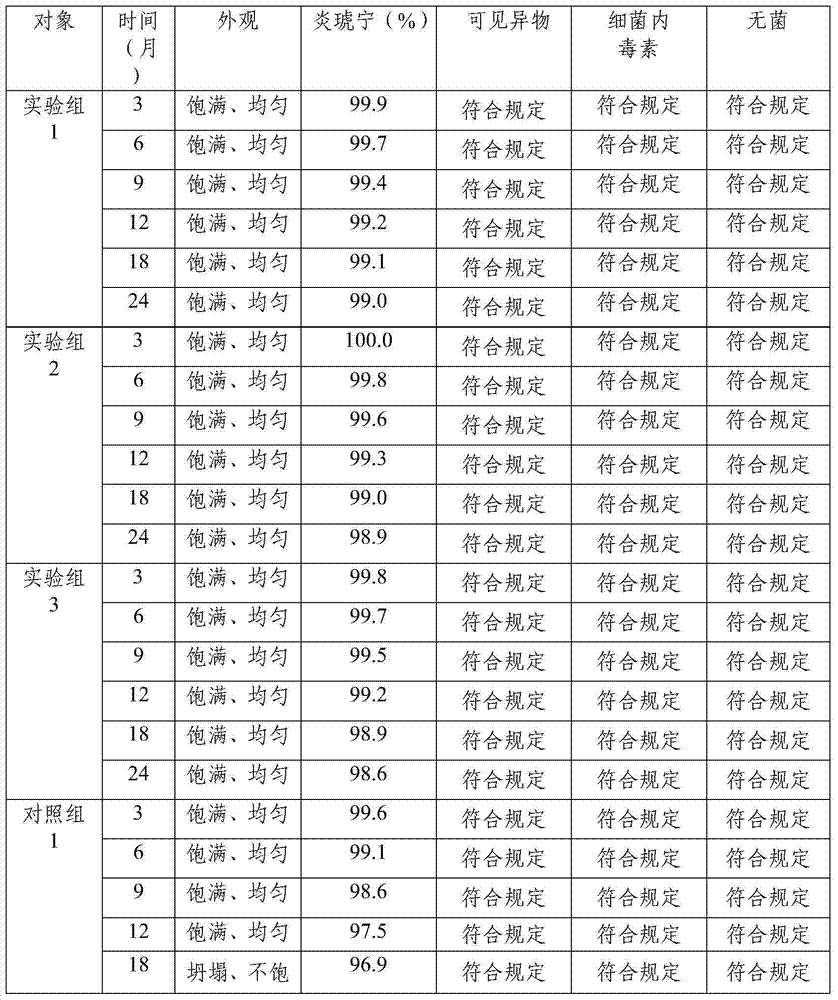
Embodiment 1
[0054] Prescription: Prepare 1000 bottles from the following ingredients: Yanhuning 80g, add water for injection to 8L;
[0055] Preparation:
[0056] (1) Weigh Yanhuning according to the prescription amount, add 75% of the prescription amount of water for injection, stir until fully dissolved, measure the pH value, and adjust the pH value to 6 with a pH regulator;
[0057] (2) Control the temperature of the solution at 10°C, add 0.1% activated carbon for needles, and stir at a speed of 64r / min for 15min;
[0058] (3) Use a 1.2 μm mixed cellulose ester microporous membrane to remove carbon, wash the activated carbon layer with the remaining 25% cold water for injection, combine the filtrate, and pass through a 0.22 μm mixed cellulose ester microporous membrane to remove carbon. bacteria;
[0059] (4) Check the content, clarity and pH value of the intermediate solution, and repackage;
[0060] (5.1) Lower the temperature of the freezer to -8°C, place the product in the box f...
Embodiment 2
[0065] Prescription: Prepare 1000 bottles from the following ingredients: Yanhuning 60g, add water for injection to 8L;
[0066] Preparation:
[0067] (1) Weigh Yanhuning according to the prescription amount, add 70% of the prescription amount of water for injection, stir well until fully dissolved, measure the pH value, and adjust the pH value to 6 with a pH regulator;
[0068] (2) Control the temperature of the solution at 5°C, add 0.05% activated carbon for needles, and stir at a speed of 60r / min for 10min;
[0069] (3) Use a 1.2 μm mixed cellulose ester microporous membrane to remove carbon, wash the activated carbon layer with the remaining 30% cold water for injection, combine the filtrate, and pass through a 0.22 μm mixed cellulose ester microporous membrane to remove carbon. bacteria;
[0070] (4) Check the content, clarity and pH value of the intermediate solution, and repackage;
[0071] (5.1) Lower the temperature of the freezer to -10°C, place the product in the...
Embodiment 3
[0076] Prescription: Prepare 1000 bottles from the following raw materials: Yanhuning 100g, add water for injection to 8L;
[0077] Preparation:
[0078] (1) Weigh Yanhuning according to the prescription amount, add 80% of the prescription amount of water for injection, stir until fully dissolved, measure the pH value, and adjust the pH value to 7 with a pH regulator;
[0079] (2) Control the temperature of the solution at 15°C, add 0.15% activated carbon for needles, and stir at a speed of 65r / min for 20min;
[0080] (3) Use a 1.2 μm mixed cellulose ester microporous membrane to remove carbon, wash the activated carbon layer with the remaining 20% cold water for injection, combine the filtrate, and pass through a 0.22 μm mixed cellulose ester microporous membrane to remove carbon. bacteria;
[0081] (4) Check the content, clarity and pH value of the intermediate solution, and repackage;
[0082] (5.1) Lower the temperature of the freezer to -5°C, place the product in the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com