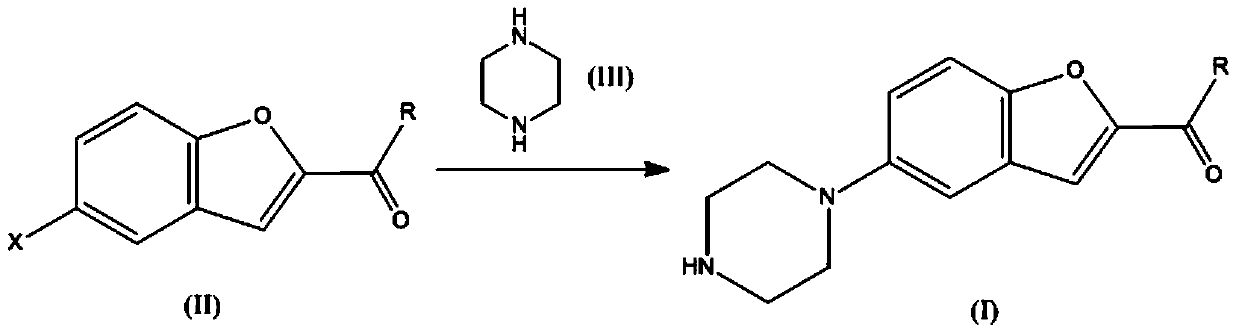
Method for preparing vilazodone intermediate 5-piperazinyl-2-acyl substituted benzofuran
A technology of acyl substitution and benzofuran, which is applied in the field of preparation of vilazodone intermediate 5-piperazinyl-2-acyl substituted benzofuran, and can solve the problem of unsubstituted 5-piperazinyl-2-acyl Preparation methods of benzofuran and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Add 5-chloro-2-amidobenzofuran (3.91g, 0.02mol), piperazine (3.44g, 0.04mol), copper chloride (0.27g, 0.002mol) into a 100ml three-necked flask, and dimethylsulfoxide (50ml), heated and stirred at 150°C for reaction. The reaction was monitored by thin-layer chromatography. After the reaction was completed, the solvent was removed, and 10 ml of ether was added to the system, and stirred vigorously, and a solid precipitated out. The solid was recrystallized in ethyl acetate to obtain 3.52 g of 5-piperazinyl-2-amidobenzofuran with a yield of 72%.
[0044] MS (ESI + , m / e): 246.12 [M+H] +
[0045] 1 H-NMR (400MHz, CDCl 3 )δ(ppm):1.91(m,1H),2.78(m,4H),3.46(m,4H),6.52(s,1H),7.78(s,1H),7.48(s,1H),7.59( s,1H),7.85(s,2H)
Embodiment 2
[0046] Example 2 Add 5-chloro-2-amidobenzofuran (3.91g, 0.02mol), piperazine (4.30g, 0.05mol), copper bromide (0.45g, 0.002mol) into a 100ml three-necked flask, and dichloromethane (50ml), heated and stirred at 150°C for reaction. The reaction was monitored by thin-layer chromatography. After the reaction was completed, the solvent was removed, and 10 ml of ether was added to the system, and stirred vigorously, and a solid precipitated out. The solid was recrystallized in ethyl acetate to obtain 3.33 g of 5-piperazinyl-2-amidobenzofuran with a yield of 68%.
Embodiment 3
[0047] Example 3 Add 5-bromo-2-amidobenzofuran (4.80g, 0.02mol), piperazine (5.16g, 0.06mol), copper cyanide (0.23g, 0.002mol) into a 100ml three-necked flask, and dimethylformamide (50ml), heated and stirred at 150°C for reaction. The reaction was monitored by thin-layer chromatography. After the reaction was completed, the solvent was removed, and 10 ml of ether was added to the system, and stirred vigorously, and a solid precipitated out. The solid was recrystallized from ethyl acetate to obtain 3.43 g of 5-piperazinyl-2-amidobenzofuran with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com