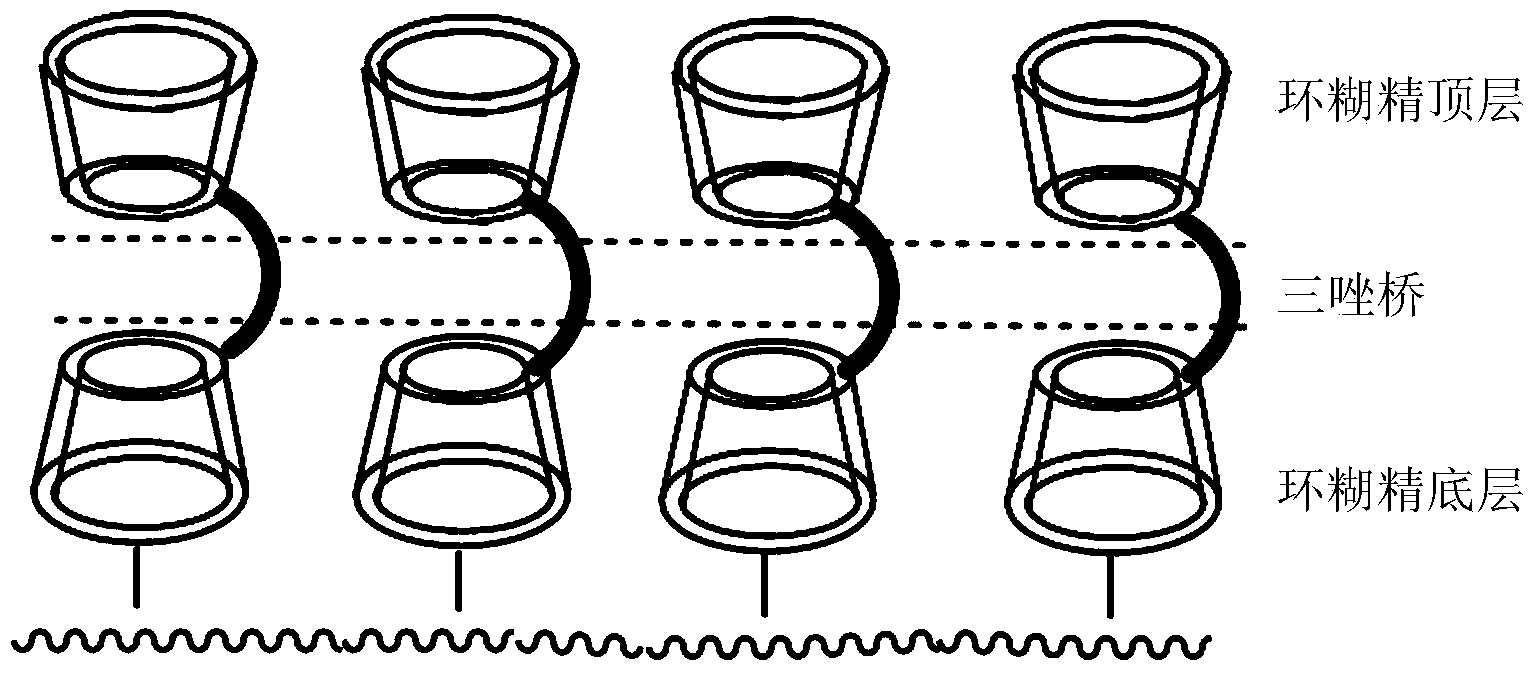
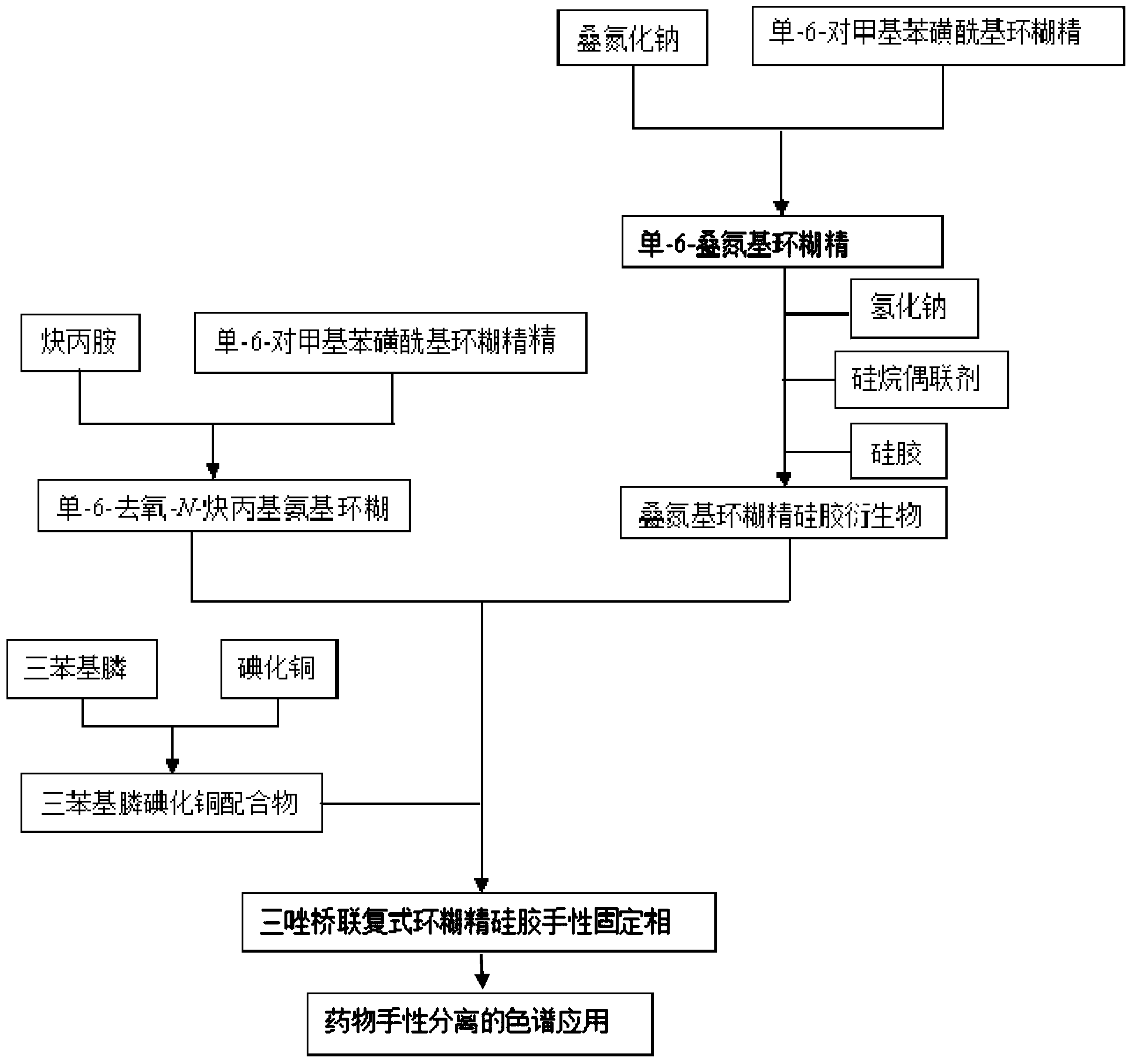
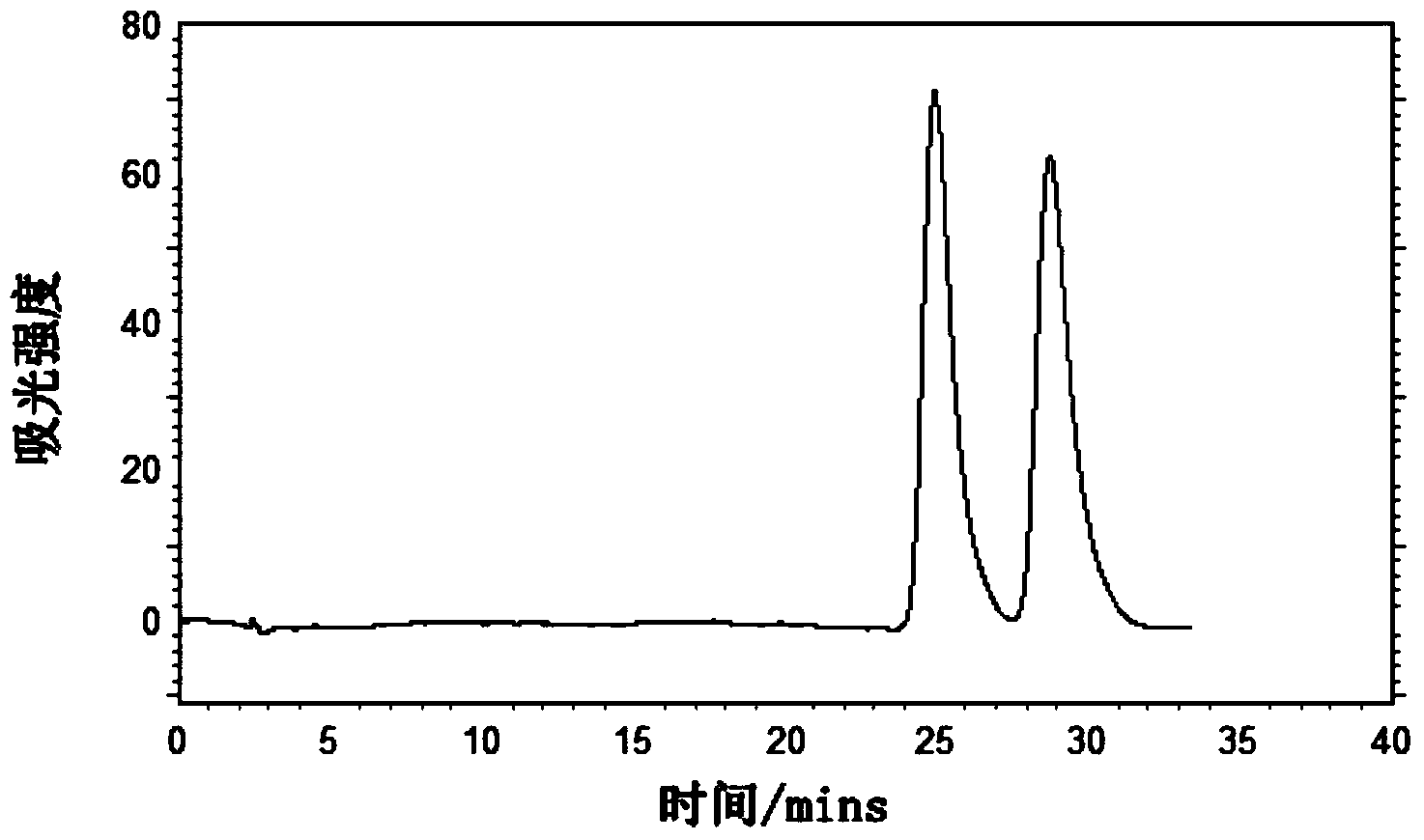
Preparation method and application of novel triazole bridging compound cyclodextrin chiral stationary phase
A chiral stationary phase, cyclodextrin technology, applied in chemical instruments and methods, other chemical processes, etc., can solve the problem of weakened enantiomeric resolution, weakened natural cyclodextrin hydroxyl hydrogen bonds, new bonding arms Development difficulties and other problems, to achieve the effect of improving separation performance and separation throughput, enhancing chiral recognition ability, and enriching structural design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] like figure 2 Shown, the preparation method of novel triazole bridged complex cyclodextrin chiral stationary phase of the present invention, the steps are as follows:
[0036] Step 1, the preparation of azide-containing monosubstituted cyclodextrin and alkynyl-containing monosubstituted cyclodextrin, including:
[0037] 1-1) Preparation of azide cyclodextrin: add mono-6-p-toluenesulfonyl cyclodextrin and sodium azide to water; the mono-6-p-toluenesulfonyl cyclodextrin used can be Mono-6-p-toluenesulfonyl-α-cyclodextrin, Mono-6-p-toluenesulfonyl-β-cyclodextrin and Mono-6-p-toluenesulfonyl-γ-cyclodextrin One of them, the molar ratio of mono-6-p-toluenesulfonyl cyclodextrin and sodium azide is 1:0.5~1:5, react at 55~110°C for 10~30 hours, and then The reaction system was poured into acetone to precipitate a solid, filtered, washed twice with acetone, and dried to obtain mono-6-azidocyclodextrin;
[0038] The structure of the resulting azide cyclodextrin can be expresse...
Embodiment 1
[0061] In a 50 mL three-necked flask under nitrogen protection, 16 mL of propargylamine was added, and then 6 g of mono-6-p-methylbenzenesulfonyl-β-cyclodextrin was added, and heated to reflux at 90° C. for 24 hours. After cooling to room temperature under nitrogen protection, the reaction system was poured into 90 mL of acetonitrile to precipitate a solid. The solid was washed twice with acetonitrile to obtain the product mono-6-deoxy-N-propargylamino-β-cyclodextrin.
[0062] Add 50 mL of distilled water into a 100 mL three-necked flask, then add 5 g of mono-6-p-methylbenzenesulfonyl-β-cyclodextrin, then add 1.26 g of sodium azide, and heat to 85° C. for 20 hours. The reaction system was poured into 250 mL of acetone to precipitate a solid. The solid was washed twice with acetone to obtain mono-6-deoxy-azido-β-cyclodextrin.
[0063] Add 50mL of anhydrous DMF to a 100mL three-necked flask, then add 1.74g of mono-6-deoxy-azido-β-cyclodextrin, then add 0.27g of sodium hydride ...
Embodiment 2
[0069] In a 5 mL three-neck flask under nitrogen protection, 16 mL of propargylamine was added, and then 6 g of mono-6-p-toluenesulfonyl-β-cyclodextrin was added, and heated to reflux at 90° C. for 24 hours. After cooling to room temperature under nitrogen protection, the reaction system was poured into 90 mL of acetonitrile to precipitate a solid. The solid was washed twice with acetonitrile to obtain the product mono-6-deoxy-N-propargylamino-β-cyclodextrin.
[0070]Add 50 mL of distilled water to a 10 mL three-necked flask, then add 5 g of mono-6-p-toluenesulfonyl-β-cyclodextrin, then add 1.26 g of sodium azide, and heat to 85° C. for 20 hours. The reaction system was poured into 25 mL of acetone to precipitate a solid. The solid was washed twice with acetone to obtain mono-6-deoxy-azido-β-cyclodextrin.
[0071] Add 5mL of anhydrous DMF to a 100mL three-necked flask, then add 1.74g of mono-6-deoxy-azido-β-cyclodextrin, then add 0.27g of sodium hydride (95%), and stir the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com