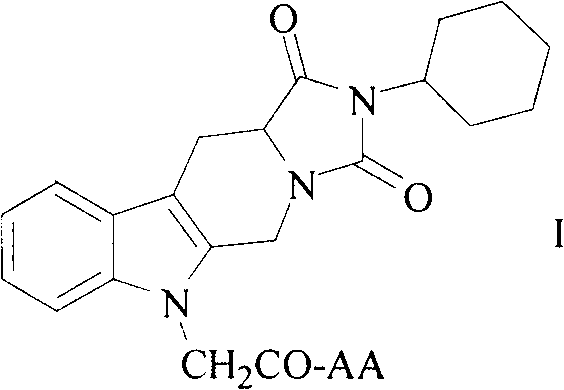
Cyclohexyl tetrahydro imidazo pyrido indole-diketone acetyl amino acids, and synthesis, antithrombotic effect and application thereof
A kind of technology of diketoacetyl and tetrahydroimidazole, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
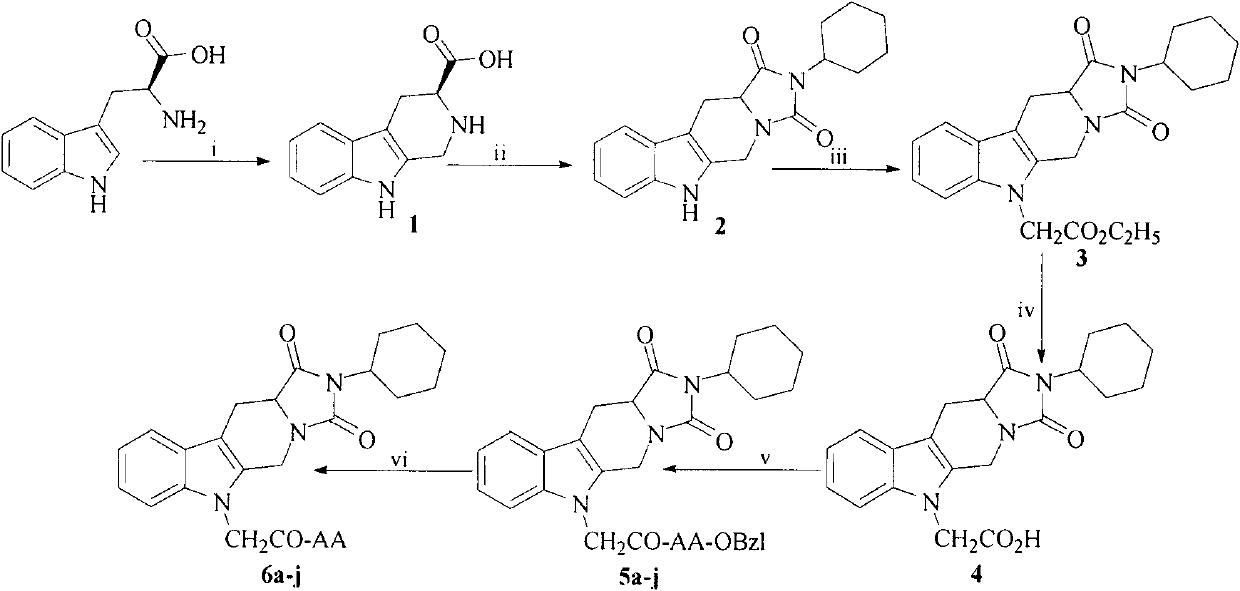
[0022] Example 1 Preparation of 3S-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (1)
[0023] While stirring, first add 5g (24.5mmol) L-tryptophan to 25ml sulfuric acid (1N), then add dropwise 75ml deionized water and 4ml (45.6mmol) formaldehyde solution (38%), the reaction solution becomes clear quickly, about 5 A large amount of solid precipitated out within minutes. After reacting for 6 hours, 8 ml of concentrated ammonia water was added dropwise to adjust the pH to 7. The reaction solution was allowed to stand overnight, filtered with suction, and the filter cake was washed with 5 ml of cold water to obtain 4.1 g (77%) of the title compound as an off-white powder. Mp 280~282℃.EI-MS 217[M+H] + . (c=0.5, CH 3 OH:HCl(1N)=1:1, v / v). 1 H-NMR (500M, DMSO-d 6 ): δ / ppm=10.99(s, 1H), 7.44(d, J=7.5Hz, 1H), 7.33(t, J=7.5Hz, 1H), 7.08(t, J=7.5Hz, 1H), 6.98 (t, J=7.5Hz, 1H), 4.30(m, 2H); 3.69(dd, J=10.5Hz, J=5.1Hz, 1H), 3.18(dd, J=10.5Hz, J=2.4Hz, 1H ), 2.83(ddd, J=10.5Hz, ...
Embodiment 2
[0024] Example 2 Preparation of 2-cyclohexyl-5,6,11,11a-tetrahydro-3,5,6,11-tetrahydro-1H-imidazo[1',5':1,6]-pyrido[ 3,4-b]indole-1,3-dione (2)
[0025] Dissolve 0.554g (2.20mmol) of 3S-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid (1) in 15ml of anhydrous DMSO and place it in an eggplant-shaped bottle under ice-bath condition , after dissolving 1.057g (5.13mmol) of DCC in 10ml of anhydrous DMSO, drop it into the above system. After the dropwise addition, the reaction solution was transferred into a microwave reaction tank, and reacted in microwave at 70°C for 6h. After the reaction was completed, cool, add water to the reaction solution, extract the aqueous layer with chloroform three times, combine the organic layers, wash with brine, dry over anhydrous sodium sulfate, filter, concentrate, silica gel column chromatography (petroleum ether / ethyl acetate=3: 1), 0.433 g (50%) of the title compound B was obtained as a yellow powder. Mp: 228-230℃; EI-MS: 323.4[M] + , 346.3...
Embodiment 3
[0026] Example 3 Preparation of 2-cyclohexyl-5,6,11,11a-tetrahydro-3,5,6,11-tetrahydro-1H-imidazo[1',5':1,6]-pyrido[ 3,4-b]indole-1,3-dione-6-ethyl acetate (3)
[0027] 1.84 g (5.70 mmol) of 2-cyclohexyl-5,6,11,11a-tetrahydro-3,5,6,11-tetrahydro-1H-imidazo[1',5':1,6]- Pyrido[3,4-b]indole-1,3-dione (2) was dissolved in 20ml of acetone, 2.36g (24mmol) of potassium carbonate and 1.3ml (11.4mmol) of ethyl bromoacetate were added and refluxed overnight. After the reaction was completed, the system was diluted with 200 ml of ethyl acetate, the ester layer was washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 2.33 g (99%) of the title compound as a pale yellow oil. ESI-MS(m / z)410[M+H] + . 1 HNMR (500M, DMSO-d 6 ): δ / ppm=7.50(d, J=7.5Hz, 1H), 7.24(d, J=3.6Hz, 2H), 7.15(m, 1H), 5.07(d, J=15.0Hz, 1H), 4.74 (s, 2H), 4.34(d, J=12.0Hz, 1H), 4.14(q, J=5.5Hz, 1H), 3.97(m, 1H), 3.38(dd,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com