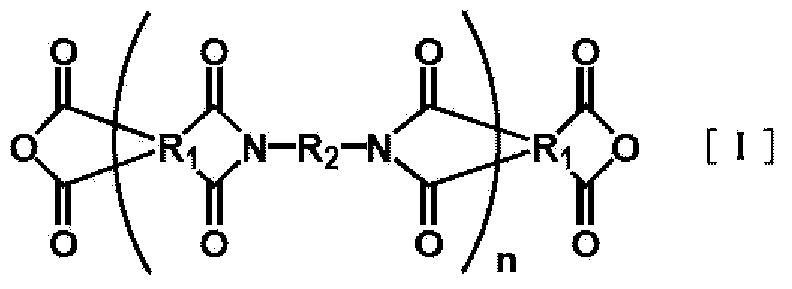
Carboxyl group-ontaining polyimide, heat-curable resin composition, and flexible metal-clad laminate
A technology of resin composition and polyimide, applied in the field of thermosetting resin composition and flexible metal-clad laminate, can solve the problems of low heat resistance, insufficient thermosetting, short circuit, etc., and achieve the effect of satisfying thermosetting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0213] Hereinafter, although an Example demonstrates this invention, this invention is not limited to these Examples. In addition, the evaluation of the characteristic value in each Example was performed by the following method.
[0214]
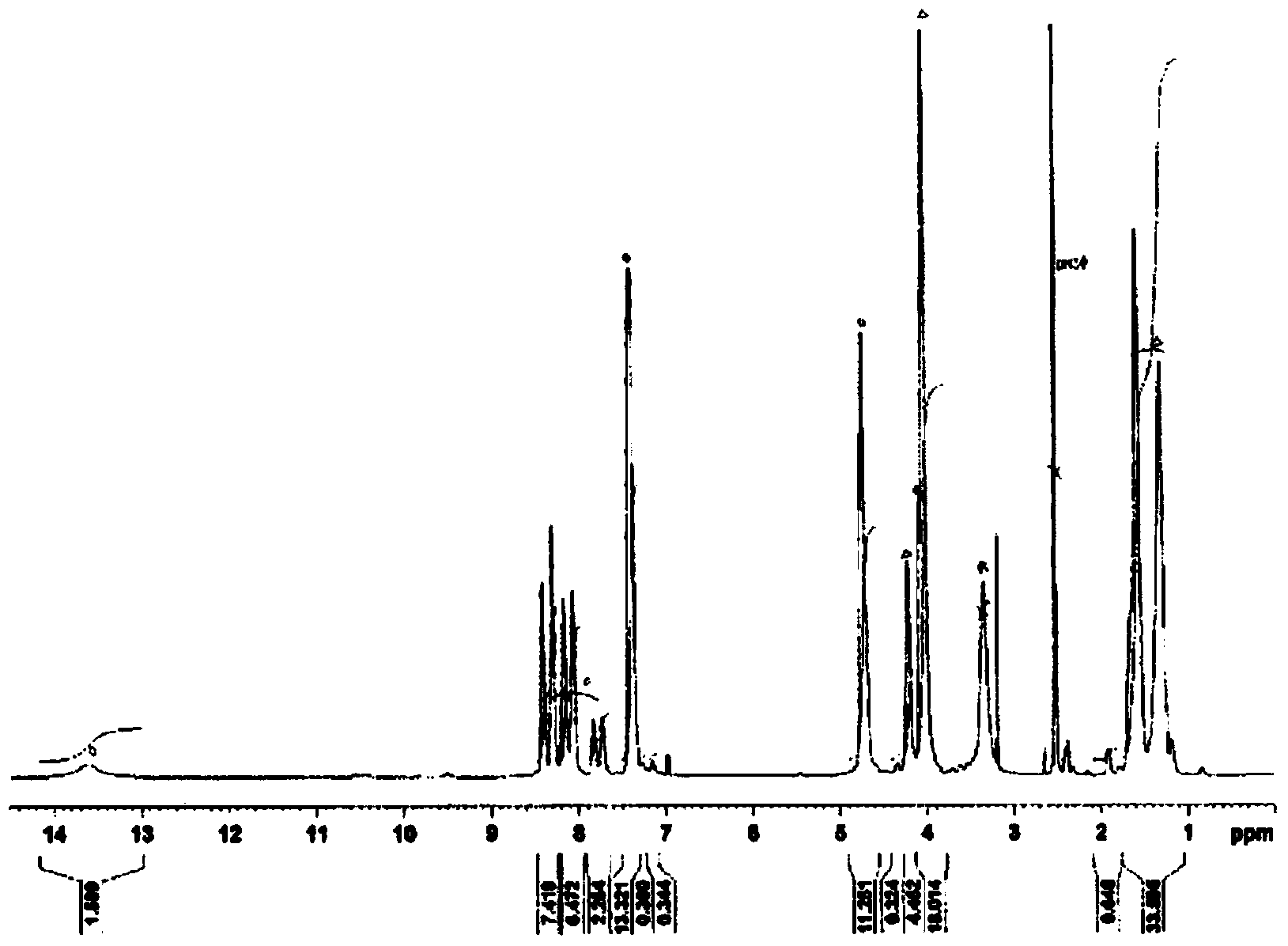
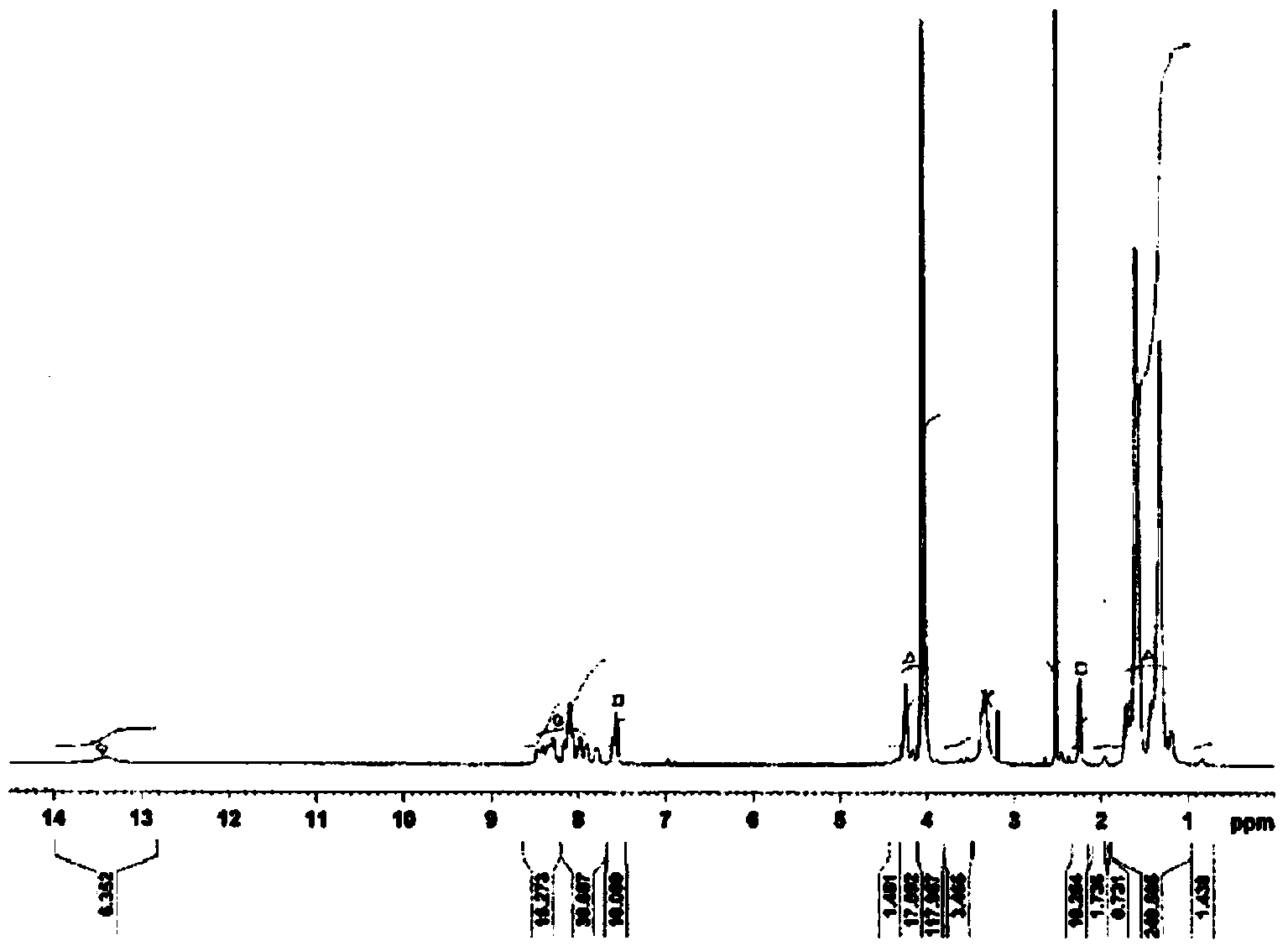
[0215] Dissolve 15 mg of a sample containing terminal anhydride group imide prepolymer and carboxyl group-containing polyimide in 0.6 ml of deuterated dimethyl sulfoxide, and use a Fourier transform nuclear magnetic resonance spectrometer (BioSpin AVANCE500 manufactured by BRUKER Corporation) )conduct 1 In H-NMR analysis, the molar ratio was obtained from the integral ratio.
[0216]
[0217] Dissolve and / or dilute samples such as imide prepolymers containing terminal anhydride groups and polyimides containing carboxyl groups with tetrahydrofuran so that the resin concentration is about 0.5% by weight. The substance obtained by filtration with a membrane filter was used as a measurement sample, and the molecular weight was measured by ...
Synthetic example 1-2、1-7
[0260] Change the composition of the raw material as shown in Table 1, dissolve the tetracarboxylic dianhydride in the solvent at 180°, and set the reaction conditions with the diisocyanate compound to 180° C. for 3 hours. In addition, the synthesis example 1- 1. Operate in the same way to obtain various carboxyl-containing polyimides. Table 1 shows the composition and physical properties of these resins. In addition, the carboxyl group-containing thermosetting polyimide (1-2) obtained in Synthesis Example 1-2 1 The H-NMR spectrum is shown in figure 2 .
Synthetic example 1-3、1-4、1-5、1-6
[0262] Various carboxyl group-containing polyimides were obtained in the same manner as in Synthesis Example 1-1, except that the composition of the raw materials was changed as shown in Table 1. Table 1 shows the composition and physical properties of these resins.
[0263] [Table 1]
[0264]
[0265] The meanings of the abbreviations in Table 1 are described below.
[0266] TMEG: Ethylene glycol bis(trimellitic anhydride) ester
[0267] PMDA: pyromellitic dianhydride
[0268] BPDA: 3,3’,4,4’-Biphenyltetracarboxylic dianhydride
[0269] BTDA: 3,3',4,4'-Benzophenone tetracarboxylic dianhydride
[0270] ODPA: 4,4'-oxydiphthalic anhydride (ODPA)
[0271] MDI: 4,4'-Diphenylmethane diisocyanate
[0272] TDI: 2,4-Toluene diisocyanate
[0273] C-2090: polycarbonate diol (3-methyl-1,5-pentanediol / 1,6-hexanediol) manufactured by Kuraray Co., Ltd., number average molecular weight about 2000
[0274] T5650E: Asahi Kasei Chemicals Co., Ltd. polycarbonate diol (1,5-pentanediol / ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com