Neohesperidin synthesis technology
A technology of neohesperidin and synthesis process, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., to achieve the effects of reducing production cost, low production cost and improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
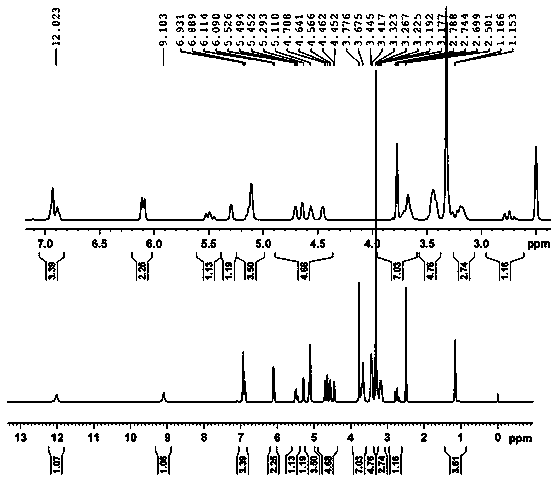
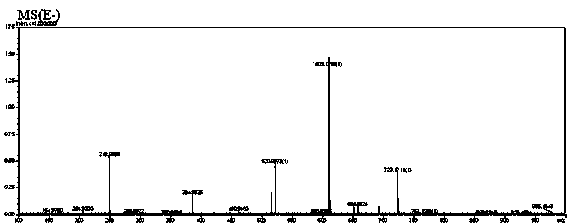
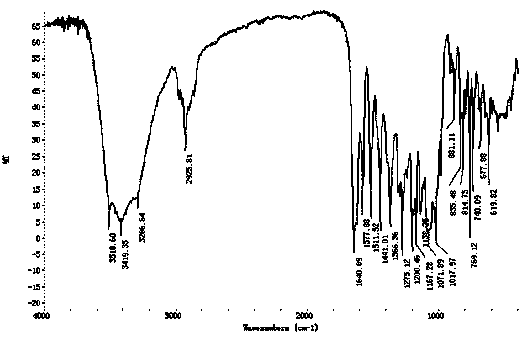
[0028] Add 3.0g (0.0063mol in molar ratio, the same below,) root bark acetophenone-4'-β-neohesperidoside and 0.95g (0.0063mol) isovanillin into 32mL of 98% ethanol, Stir to dissolve, pass N 2 After getting rid of the air in the bottle, raise the temperature, add 0.15g (0.0021mol) tetrahydropyrrole, then add 0.13g (0.0022mol) acetic acid, reflux at 95°C, stop the N 2 , and counting from the beginning of reflux, reflux and stirring reaction for 5 hours, then stop heating, cool to room temperature, filter the filter cake with a small amount of hot ethanol until colorless, and vacuum dry at 50°C to obtain 3.26g off-white powdery solid substance The yield of neohesperidin is 85%, and the purity of the product determined by liquid chromatography is 99.3%.
Embodiment 2
[0030] Add 3.0g (0.0063mol) of acetophenone-4’-β-neohesperidoside and 0.95g (0.0063mol) of isovanillin into 35mL of 98% ethanol, stir to dissolve, pass N 2 After getting rid of the air in the bottle, heat up, add 0.22g (0.0031mol) tetrahydropyrrole, and then add 0.15g (0.0025mol) acetic acid, reflux at 95°C, count from the beginning of reflux, reflux and stir for 4 hours, then stop heating. Cool to room temperature, filter through a Bush funnel, wash the filter cake with a small amount of hot ethanol until it is colorless, and dry it under vacuum at 50°C to obtain 3.30 g of off-white powdery solid neohesperidin, with a yield of 86%. The product was determined by liquid chromatography The purity is 98.6%.
Embodiment 3
[0032] Add 3.0g (0.0063mol) of acetophenone-4’-β-neohesperidoside and 0.95g (0.0063mol) of isovanillin into 38mL of 98% ethanol, stir to dissolve, pass N 2 After getting rid of the air in the bottle, raise the temperature, add 0.26g (0.0037mol) tetrahydropyrrole, and then add 0.10g (0.0017mol) acetic acid, reflux at 95°C, stop the N 2 , and from the start of reflux timing, reflux stirring reaction for 4 hours, then stop heating, cool to room temperature, filter the filter cake with a small amount of hot ethanol until it is colorless, and vacuum dry at 50°C to obtain 3.30g off-white powdery solid substance The yield of neohesperidin is 86%, and the purity of the product as determined by liquid chromatography is 98.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com