Preparation method of synergistic tylosin tartrate soluble powder compound medicine
A technology of tylosin tartrate and compound prescription is applied in the field of compound medicine preparation of synergistic tylosin tartrate soluble powder, which can solve the problems of incomplete curative effect and the like, and achieves long drug effect maintenance time, fast onset of effect, and storage. long term effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
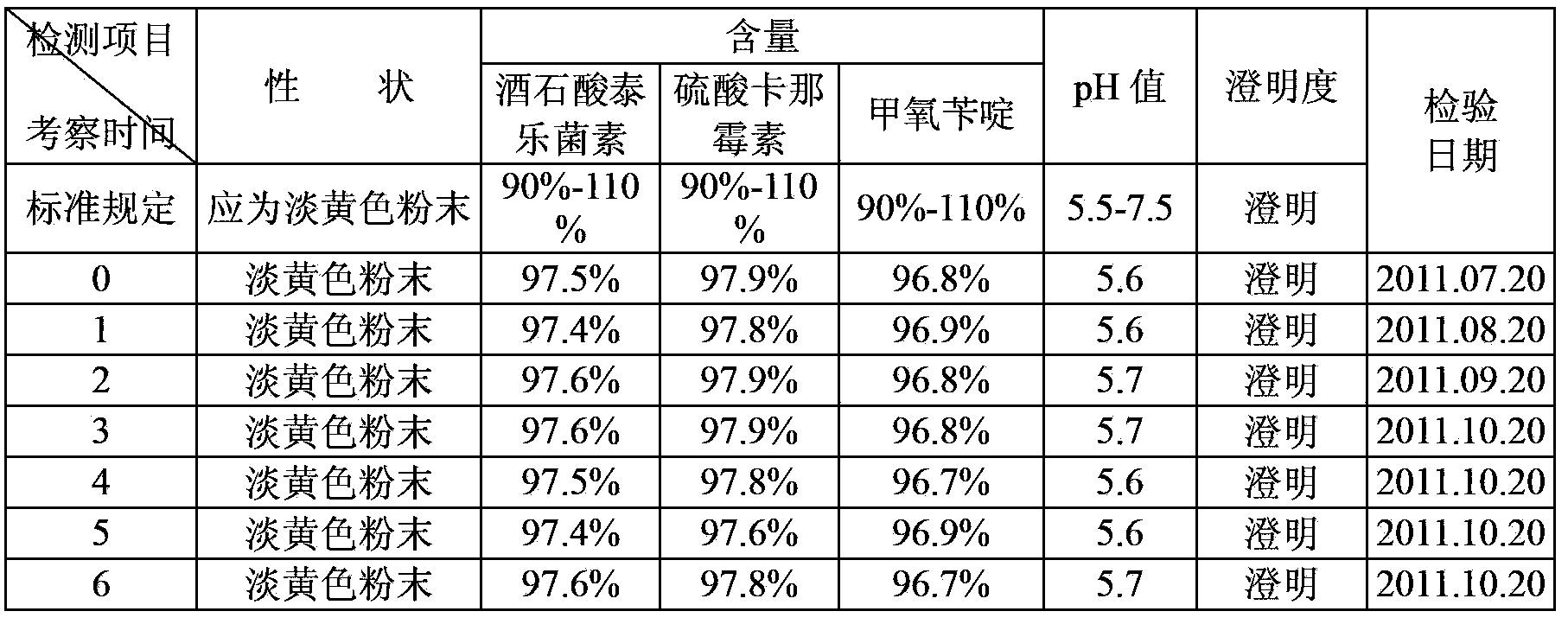
[0032] Tylosin tartrate 1g, kanamycin sulfate 1g, trimethoprim 0.2g, citric acid 0.2g, procaine hydrochloride 0.05g.
[0033] Preparation:
[0034] Respectively take trimethoprim and citric acid of the prescribed amount, mix them and pulverize them through a flow energy mill, pass through a 120 mesh sieve, and then deliver them in equal amounts with tylosin tartrate, kanamycin sulfate, and procaine hydrochloride. Addition, mixing evenly, inspection of semi-finished products, packaging, packaging, inspection of finished products, that is to say.
Embodiment 2
[0036] Tylosin tartrate 1g, kanamycin sulfate 1g, trimethoprim 0.2g, succinic acid 0.2g, procaine hydrochloride 0.05g.
[0037] Preparation:
[0038] Weigh respectively the prescribed amount of trimethoprim and succinic acid, mix them and pulverize them through a flow energy mill, pass through a 120-mesh sieve, and then add them with tylosin tartrate, kanamycin sulfate, and pain relieving agents in equal amounts. Mix evenly, inspect the semi-finished product, subpackage, pack, inspect the finished product, and put it into storage.
Embodiment 3
[0040] Tylosin tartrate 1g, kanamycin sulfate 1g, trimethoprim 0.2g, citric acid 0.2g, lidocaine hydrochloride 0.05g.
[0041] Preparation:
[0042] Respectively weigh trimethoprim and citric acid in the prescribed amount, mix them, pass through a flow energy mill, pass through a 150-mesh sieve, and then mix them with tylosin tartrate, kanamycin sulfate, and pain relieving agents in equal increments Uniformity, inspection of semi-finished products, sub-packaging, packaging, inspection of finished products, warehousing, and ready to go.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com