O-hydroxyl-imidogen containing all-conjugated visible light sensitizer with Y-shaped structure and synthesis thereof
An ortho-hydroxyl and visible light technology, applied in the preparation of imino compounds, organic chemistry, etc., can solve the problem of rare photosensitizers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (4,4’-bis((2”-hydroxy-phenyl-methyl-imino)-styryl))-triphenylamine
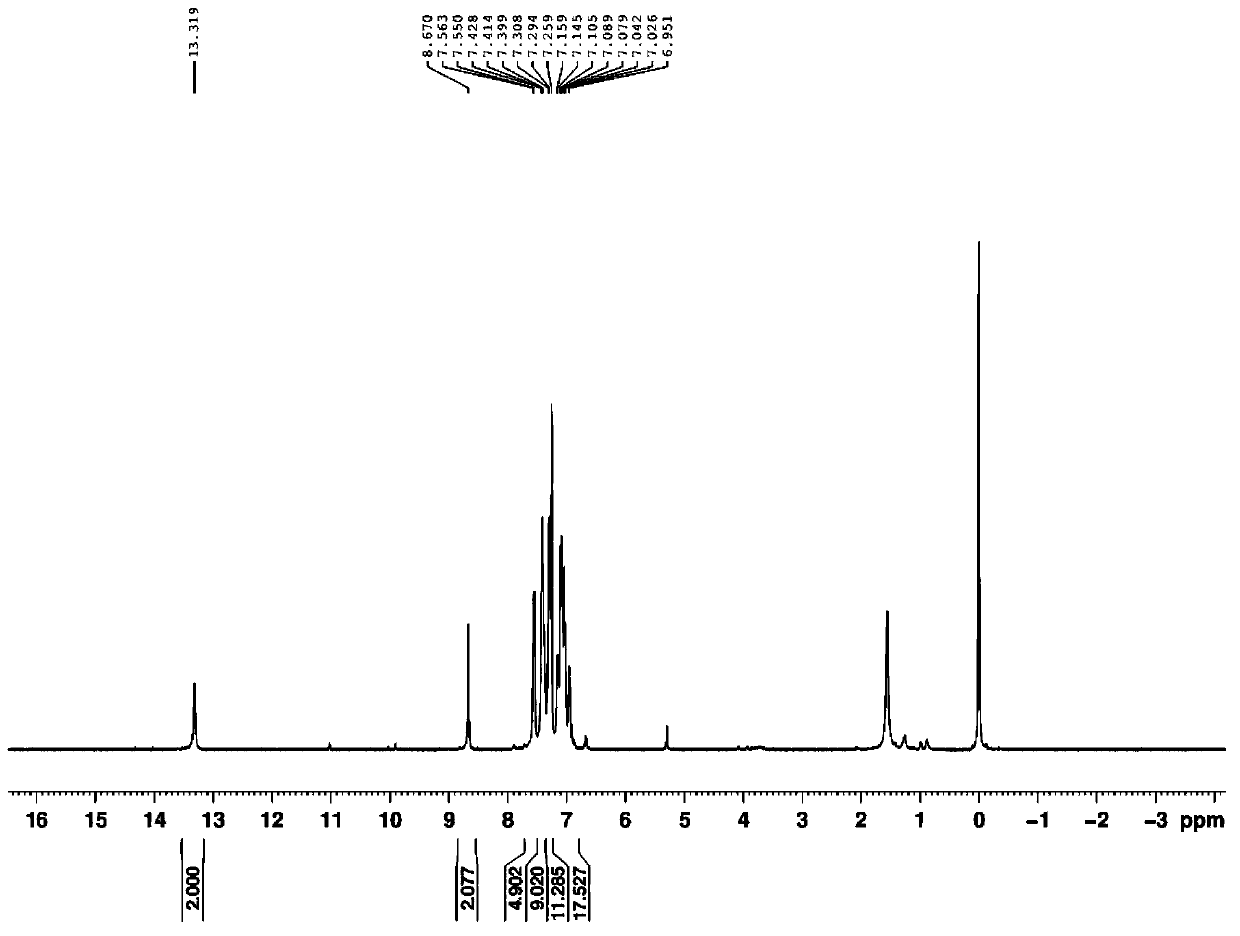
[0021] Mix 4,4'-bis((4"-amino)-styryl)-triphenylamine (5mmol, 2.395g) with o-hydroxy-benzaldehyde (10mmol, 1.22g, 1.04ml) in a three-necked glass bottle , with 50ml of ethanol as solvent, magnetic stirring at room temperature for 10 hours to end the reaction, spin-drying under reduced pressure to obtain the crude product, using benzene as the eluent, and separating the pure product with silica gel chromatography column. The yield is 50%, and the melting point: 275~ 277°C. Its 1 The H NMR spectrum is as figure 1 as shown, 1 H-NMR (D 1 -CDCl 3 ,500MHz)δ(ppm):13.319(s,-OH,2H),8.670(s,N=CH,2H),7.563-7.550(d,Ar-H,4H),7.428-7.399(t,Ar- H, 8H), 7.308-7.294 (d, Ar-H, 6H), 7.159-6.951 (m, Ar-H, 11H; CH=CH, 4H).
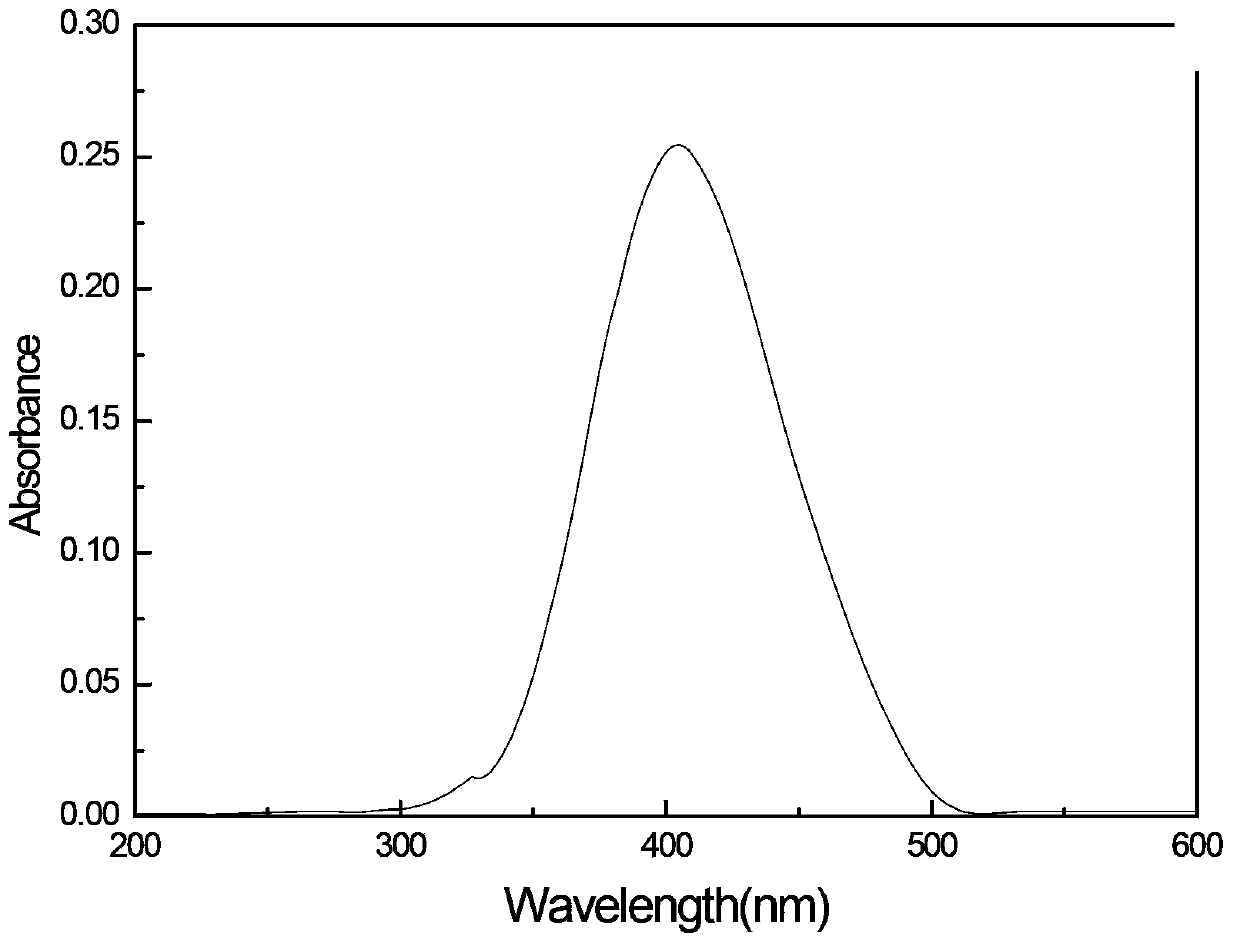
[0022] The preparation concentration is 1×10 -5 mol·l -1 Ethyl acetate solution of (4,4'-bis((2"-hydroxy-phenyl-methyl-imino)-styryl))-triphenylamine, using UV-visible spectrometer, scanning 20...
Embodiment 2
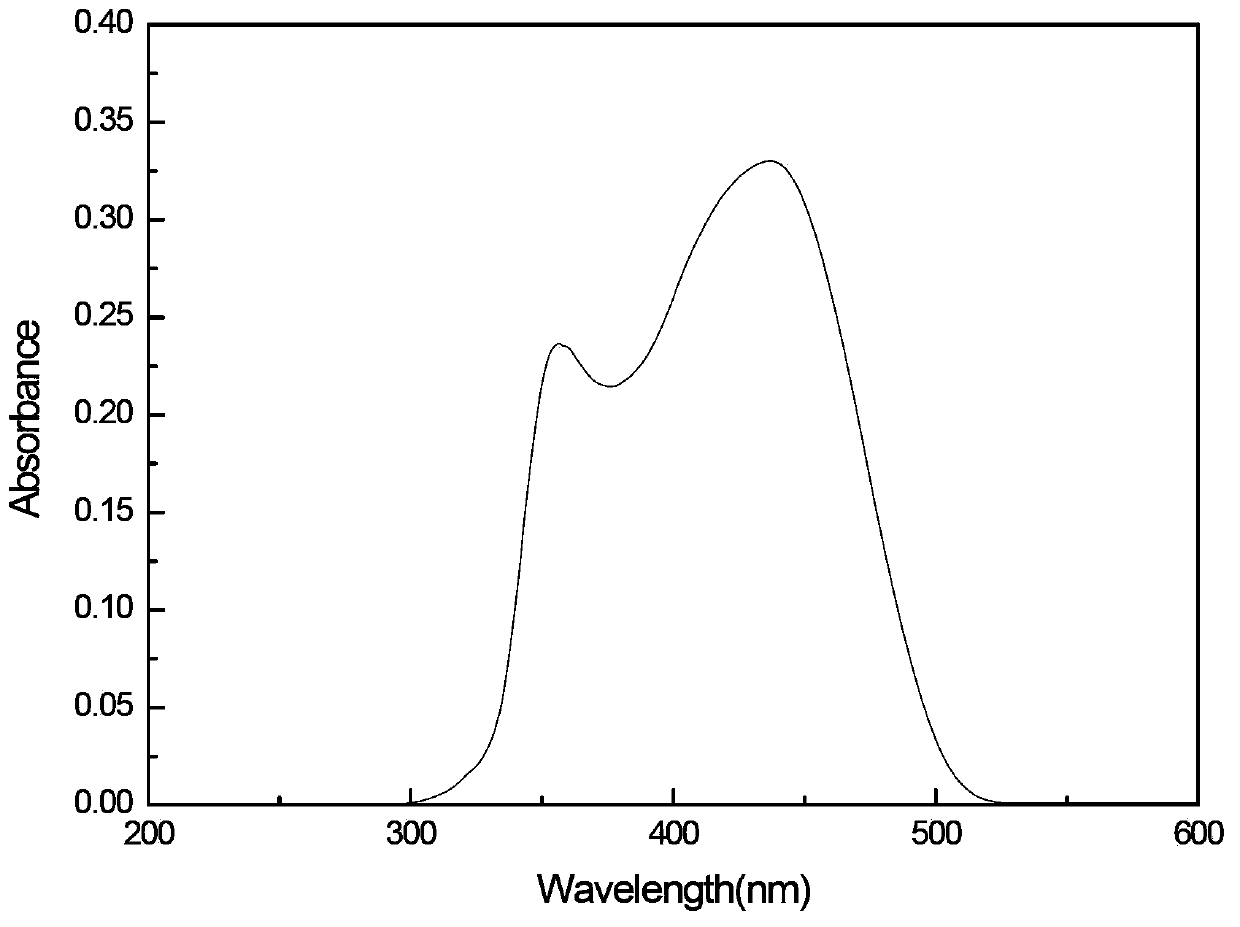
[0024] The preparation concentration is 1×10 -5 mol·l -1 (4,4'-bis((2"-hydroxy-4''-(N,N-dimethyl)-phenyl-methyl-imino)-styryl))-triphenylamine acetic acid Ethyl ester solution, using a UV-visible spectrometer, scans the UV-visible absorption spectrum of 200nm~600nm. Its UV-visible absorption spectrum is as follows image 3 As shown, the maximum absorption wavelength is 445nm, and the absorption peak extends to 520nm.
Embodiment 3
[0026] The preparation concentration is 1×10 -5 mol·l -1 (4,4'-bis((2"-hydroxy-4''-(N,N-diethyl)-phenyl-methyl-imino)-styryl))-triphenylamine acetic acid Ethyl ester solution, using a UV-visible spectrometer, scans the UV-visible absorption spectrum of 200nm~600nm. Its UV-visible absorption spectrum is as follows Figure 4 As shown, the maximum absorption wavelength is 476nm, and the absorption peak extends to 550nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com