Cathode material and lithium ion battery therefrom
一种阴极材料、阳极材料的技术,应用在电池电极、二次电池、电路等方向,能够解决降低容量等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Preparation of cathode material
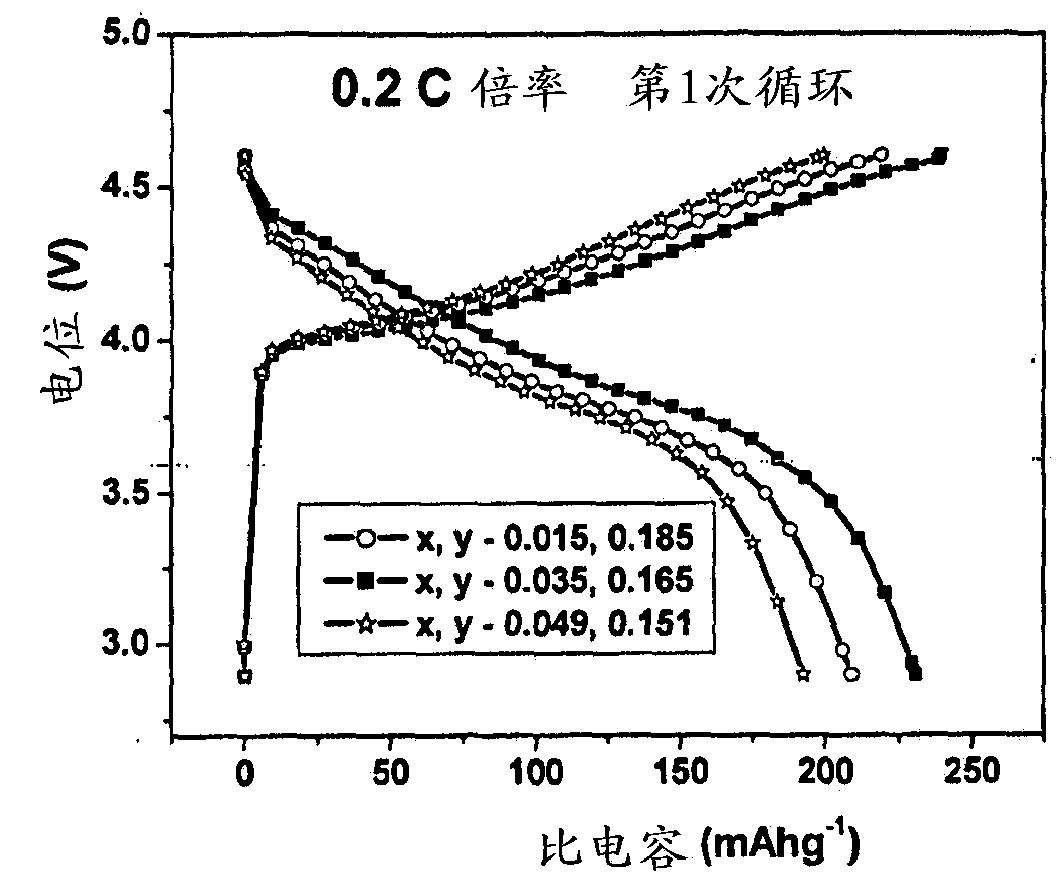
[0100] LiMg 0.015 Cu 0.185 co 0.8 o 2 preparation of
[0101]LiMg was carried out by x Cu y co 1-x-y o 2 Synthesis of (0≤x,y≤0.2) materials: mixing stoichiometric amounts of anhydrous LiNO 3 (6.895g), Cu(NO 3 ) 2 .3H 2 O(4.469g), Mg(NO 3 ).6H 2 O(0.3846g) and Co(NO 3 ) 2 .4H 2 O (23.283g), then dissolved in 100ml triple distilled water. The resulting metal ion solution was continuously stirred at 300 rpm for 2 h under warm conditions (100 °C). The above concentrated solution was transferred to a porcelain dish and placed in the center of a microwave oven turntable (Kenstar, India 2450MHz, 1500W). The solution was irradiated for 35 minutes at full rated power (100% microwave power (2450 MHz microwave frequency)). During the reaction, the chemical components are heated rapidly, and a red glow appears within the disk throughout the reaction. After the reaction was complete, the product was dried in an oven for two hours...
Embodiment 2
[0103] Preparation of cathode material
[0104] LiMg 0.035 Cu 0.165 co 0.8 o 2 preparation of
[0105] LiMg was carried out by x Cu y co 1-x-y o 2 Synthesis of (x,y≥1) materials: mixing stoichiometric amounts of anhydrous LiNO 3 (6.895g), Cu(NO 3 ) 2 .3H 2 O(3.9864g), Mg(NO 3 ).6H 2 O(0.8674g) and Co(NO 3 ) 2 .4H 2 O (23.283g), then dissolved in triple distilled water (100ml). The resulting metal ion solution was continuously stirred under warm conditions (100 °C) for 2 hours (300 rpm). The above concentrated solution was transferred to a porcelain dish and placed in the center of a microwave oven turntable (Kenstar, India 2450MHz, 1500W). The solution was irradiated for 35 minutes at full rated power (100% microwave power (2450 MHz microwave frequency)). During the reaction, the chemical components are heated rapidly, and a red glow appears within the disk throughout the reaction. After the reaction was complete, the product was dried in an oven for two ho...
Embodiment 3
[0107] Preparation of cathode material
[0108] LiMg 0.049 Cu 0.151 co 0.8 o 2 preparation of
[0109] LiMg was carried out by x Cu y co 1-x-y o 2 Synthesis of (x≥0.1,y≤0.1) materials: mixing stoichiometric amounts of anhydrous LiNO 3 (6.895g), Cu(NO 3 ) 2 .3H 2 O(3.648g), Mg(NO 3 ).6H 2 O(1.2564g) and Co(NO 3 ) 2 .4H 2 O (23.283g), then dissolved in 100ml triple distilled water. The resulting metal ion solution was continuously stirred under warm conditions (100 °C) for 2 hours (300 rpm). The above concentrated solution was transferred to a porcelain dish and placed in the center of a microwave oven turntable (Kenstar, India 2450MHz, 1500W). The solution was irradiated for 30-40 minutes at full rated power (100% microwave power (2450 MHz microwave frequency)). During the reaction, the chemical components are heated rapidly, and a red glow appears within the disk throughout the reaction. After the reaction was complete, the product was dried in an oven for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| flexural strength | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com