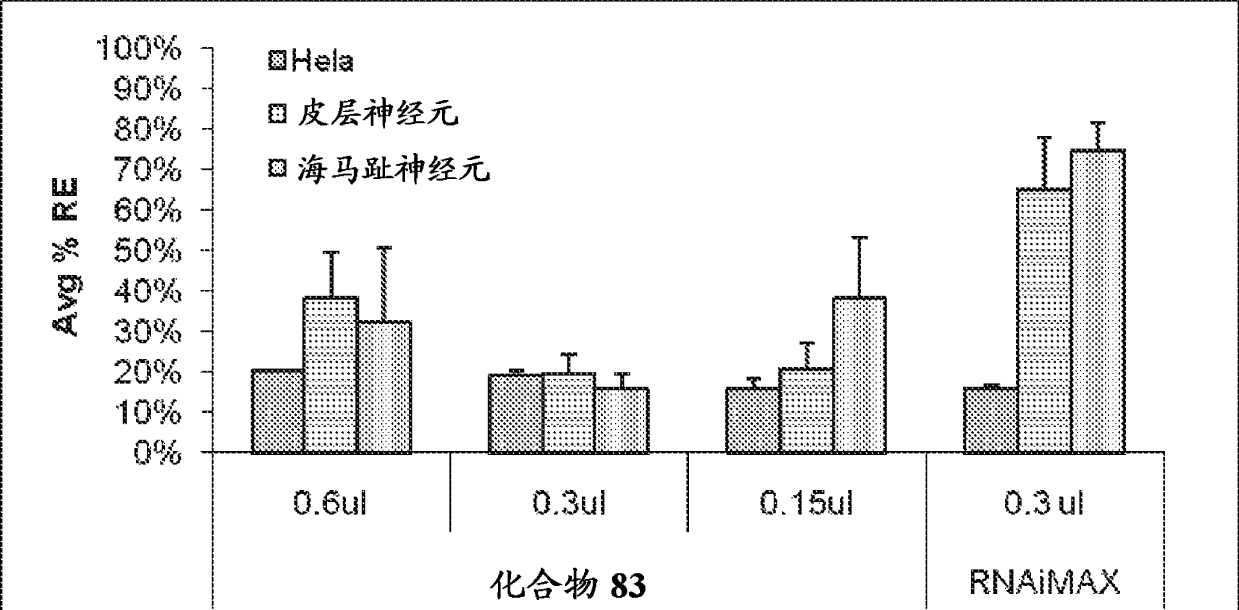
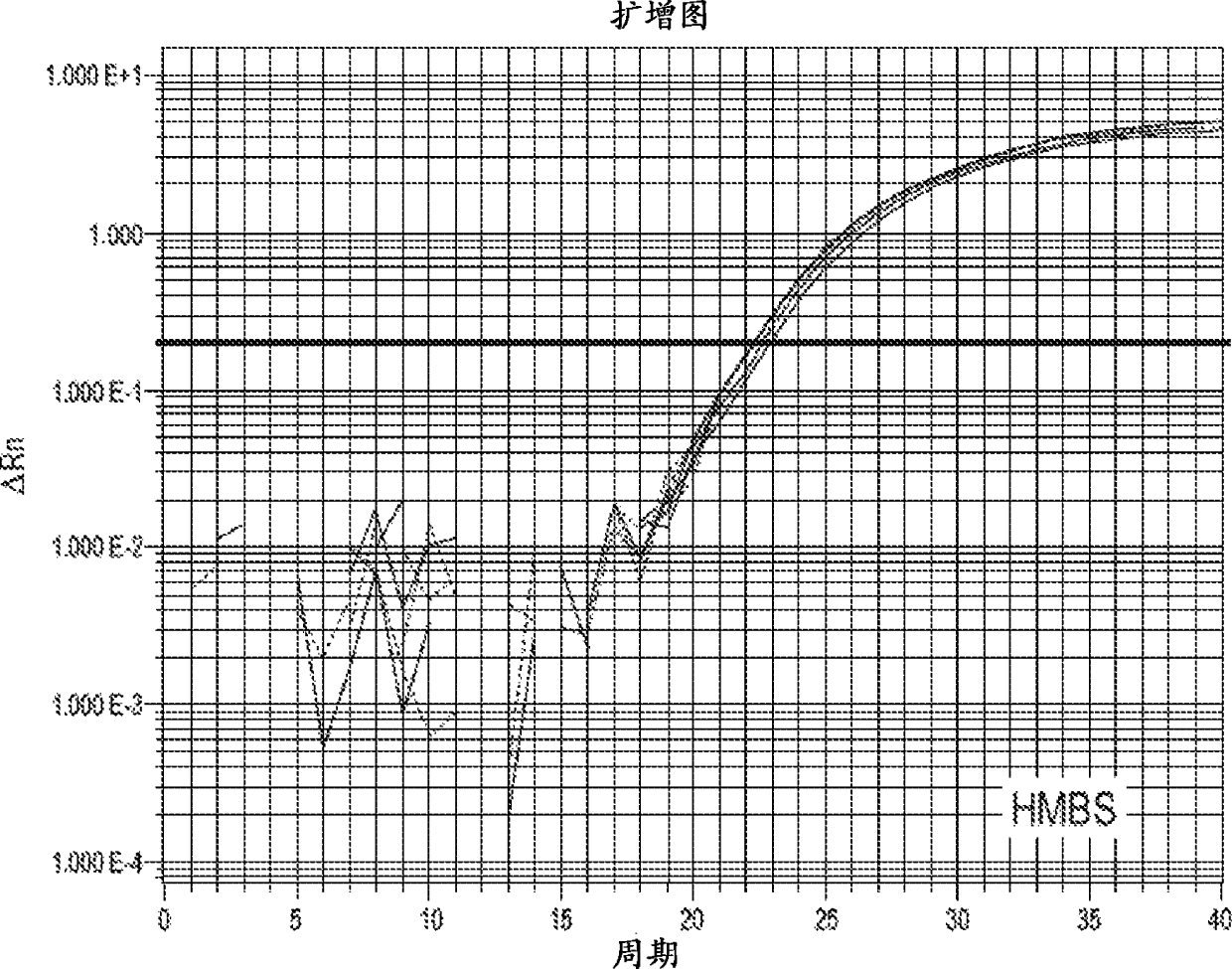
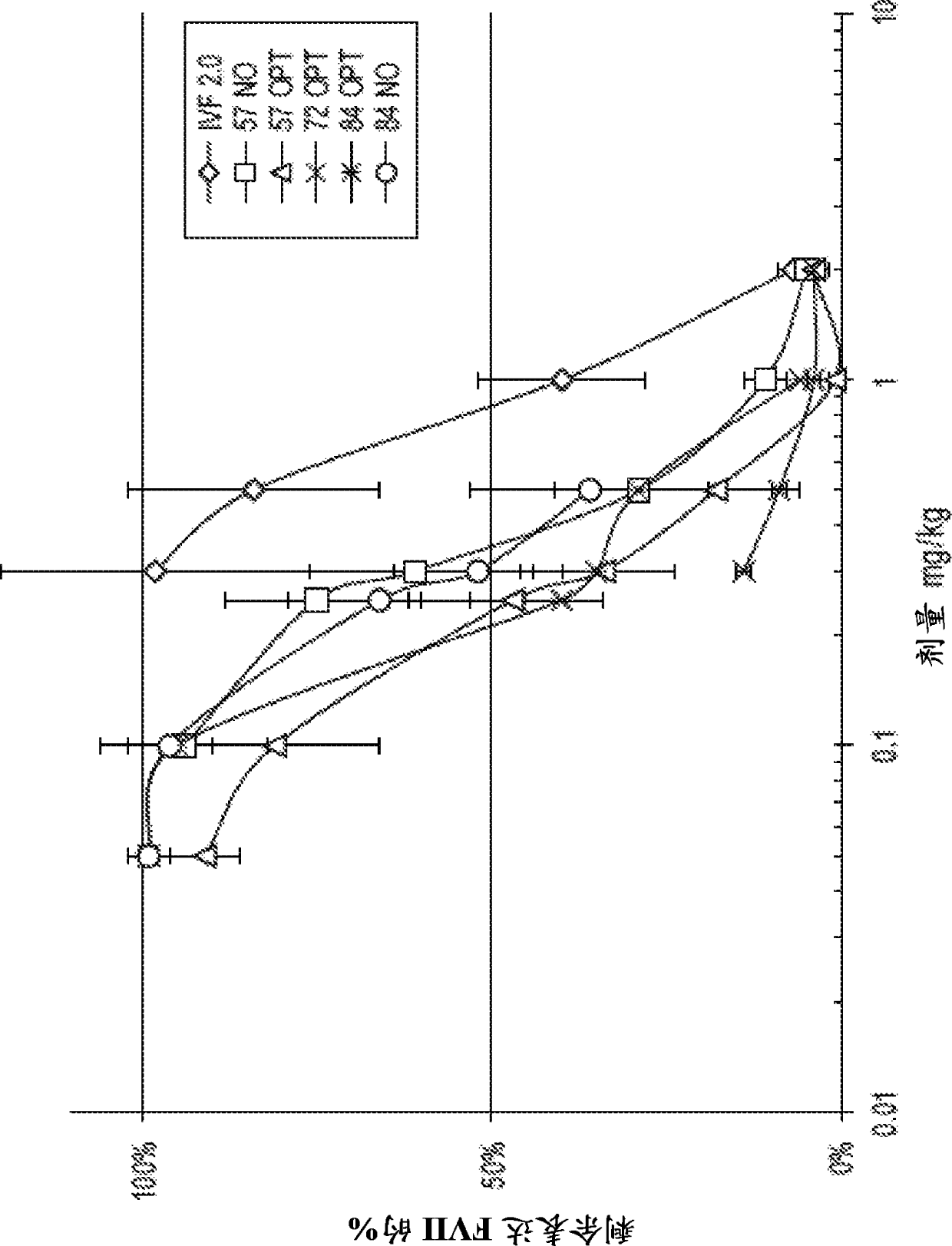
Amine-containing transfection reagents and methods for making and using same
A compound, pharmaceutical technology, applied in the field of transfection complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0104] Amine-containing transfection compounds
[0105] The present invention describes various amine-containing compounds useful as transfection reagents and methods for their synthesis. More specifically, according to some embodiments of the present invention, there is provided a compound having the general structure I or a pharmaceutically acceptable salt thereof:
[0106]
[0107]
[0108] where X 1 and x 2 Each of is a moiety independently selected from O, S, N-A and C-A, wherein A is selected from hydrogen and C 1 -C 20 Hydrocarbon chain; each of Y and Z is a moiety independently selected from CH-OH, C=O, C=S, S=O and SO 2 ; 1 , R 2 , R 3 , R 4 , R 5 , R 6 and R 7 Each of is a moiety independently selected from hydrogen, cyclic or acyclic, substituted or unsubstituted, branched or linear aliphatic group, cyclic or acyclic, substituted Substituted or unsubstituted, branched or straight chain heteroaliphatic, substituted or unsubstituted, branched or st...
Embodiment 1
[0242] The synthesis of embodiment 1.2-bromo-N-dodecylacetamide
[0243] The title intermediate was synthesized according to the following general reaction scheme:
[0244]
[0245] In a round bottom flask with a pressure equilibrating addition funnel, to 200 mL of dichloromethane was added 1.5 g (7.431 mmol, 1.05 equiv) of bromoacetyl bromide and the mixture was stirred under nitrogen. The flask was cooled to about 0°C using an ice bath. 1.31 g (7.077 mmol) of dodecylamine and 0.716 g (983 μL, 7.077 mmol) of triethylamine were dissolved in 100 mL of dichloromethane. Transfer the solution to an addition funnel and slowly add the bromoacetyl solution. reverse the The mixture was stirred at about 0°C for about another 1 hour, slowly warming to room temperature by allowing the ice to melt.
[0246] The resulting reaction mixture was transferred to a separatory funnel and extracted with 100 mL of saturated sodium bicarbonate solution. The aqueous layer was washed with...
Embodiment 2
[0247] Example 2. Synthesis of tetradecyl-2-bromoacetate
[0248] The title intermediate was synthesized according to the following general reaction scheme:
[0249]
[0250] Dissolve 3 g (1.3 mL, 14.862 mmol, 1.05 equiv) of bromoacetyl bromide in 50 mL of dichloromethane. Using an ice bath, the flask was cooled to about 0°C. Dissolve 3.03 g (14.154 mmol) of tetradecyl alcohol in 50 mL of dichloromethane, and slowly add to the above mixture via a pipette, then add about 5 drops of concentrated sulfuric acid, and stir at about 0 ° C for about 30 minutes, then Stir overnight at room temperature.
[0251] Add about 150 mL of saturated sodium bicarbonate solution and stir rapidly for about 30 minutes. The mixture was then transferred to a separatory funnel, and the organic layer was collected. The aqueous layer was washed with about 100 mL of dichloromethane. The combined organic layers were dried over sodium sulfate, filtered, and concentrated to afford 4.095 g (89.7% y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com