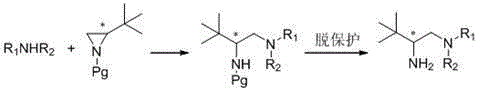
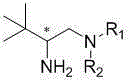
A class of chiral amine compounds derived from tertiary leucine and its preparation method and application
A technology for tertiary leucine and compounds, which is applied in the field of preparation of chiral amine compounds, can solve problems such as poor diastereoselectivity, poor reaction universality, and limited few reactions, and achieve high-efficiency catalytic performance and good controllability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0047] The preparation of a chiral amine compound derived from tert-leucine comprises the following steps:
[0048] (1) Dissolve 0.68g (8.0 mmol) of piperidine in 40 mL of anhydrous acetonitrile, and add 2.27 g (8.0 mmol) of ( S )-N-p-nitrobenzenesulfonyl-1-tert-butyl-cycloethyleneimine in 40ml of acetonitrile solution, stirred at 20°C for 36 hours, TLC detected that the reaction was complete; the mixture was concentrated under reduced pressure, and the residue was chromatographed on a silica gel column (petroleum Ether and ethyl acetate, their volume ratio = 3:1) were separated to obtain 2.80 g of white solid, and the yield was 95%.
[0049] (2) Dissolve 1.85g (5.0mmol) of the white solid obtained in step (1) in 30mL DMF, add potassium carbonate (2.07g, 15.0mmol molar concentration), thioglycolic acid (0.92g, 10.0mmol), and mix at 50°C The mixture was stirred for 12 hours, and TLC detected that the reaction was complete; the mixture was added with 100 mL of ethyl acetate, wa...
preparation Embodiment 2
[0055] The difference between Preparation Example 2 and Preparation Example 1 is that the amine used in step (1) is N-Ts protected 1,2-diphenylethylenediamine, the temperature used is 100°C, and the amine used in step (2) The temperature is 80°C, other preparations and conditions are the same as in Example 1, the final product is a white solid chiral primary amine product, and the product configuration is R , R , S ; 1 for hydrogen, R 2 It is 1,2-diphenylethylamine protected by N-Ts, and its structural formula is as follows:
[0056] .
[0057] Melting point 120-121°C; 1 HNMR (400MHz, CDCl 3 ):d7.41-7.39(m,2H),7.16-7.15(m,3H),7.06-7.03(m,5H),6.96-6.94(m,4H),4.33(d, J =8.0Hz,1H),3.64(d, J =8.8Hz,1H),2.66(dd, J =11.0,2.5Hz,1H),2.52(d, J =12.3Hz,4H),2.35(s,3H),2.28(dd, J =12.3,2.8Hz,3H),0.94(9H,s). 13 CNMR (100MHz, CDCl 3 ):d142.7, 139.6, 138.4, 137.3, 129.1, 128.3, 127.9, 127.6, 127.5, 127.4, 127.2, 127.1, 68.5, 63.3, 54.5, 49.1, 45.1, 24.6, 23.4, 22.0, 21.4. HRM...
preparation Embodiment 3
[0059] The difference between Preparation Example 3 and Preparation Example 1 is that the amine used in step (1) is cyclohexylamino alcohol, the temperature used is 20°C, the temperature used in step (2) is 20°C, and other preparation steps and conditions are the same Preparation Example 1. The final product is a colorless oily chiral primary amine product, and the product configuration is R , R , S ; 1 for hydrogen, R 2 For o-hydroxycyclohexane, its structural formula is as follows:
[0060] .
[0061] 1 HNMR (400MHz, CDCl 3 ):δ3.40(m,1H),2.82(dd, J =2.4,8.8Hz,1H),2.40(dd, J =2.4,8.4Hz,1H),2.35(td, J =3.6,10.4Hz,1H),2.16(t, J =11.2Hz,1H),1.85(d, J =12.0Hz, 2H), 1.67-1.71(m, 2H), 1.55-1.59(m, 1H), 1.08-1.24(m, 4H), 0.85(s, 9H). 13 CNMR (100MHz, CDCl 3 ): δ60.4, 57.0, 48.5, 33.9, 33.6, 33.5, 26.2, 26.2, 25.1, 25.0. HRMS (ESI): Theory (M+H) + C 12 h 27 N 2 O215.2123, yielding m / z 215.2125.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com