Long-acting HIV-1 (Human Immunodeficiency Virus-1) membrane fusion inhibitor
A single, high-molecular technology, applied in the field of long-acting membrane fusion polypeptide drugs, can solve the problems of loss of activity of HIV membrane fusion inhibitors and limited long-acting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Construction and expression of ABT clone
[0029] 1.1 Materials
[0030] PCR reagents: PrimeSTAR DNA Polymerase (Takara), 10X buffer (Mg+) (Takara), dNTP (Takara).
[0031] Sterilized water: high pressure deionized water.
[0032] PCR primers: synthesized by Shanghai Jieli Company.
[0033] Plasmid: PHFT plasmid was donated by Beijing Huajin Ruiqing Company, and PGEX‐6P‐1MD1.1‐L35‐CP38 plasmid was constructed by our laboratory.
[0034] Restriction enzymes: BamHI, XhoI, BglII (Takara)
[0035] T4 ligase: purchased from Takara Company.
[0036] 30% Bis-Acr polyacrylamide gel: purchased from Bio-rad.
[0037] Ni purification column: purchased from Qiagen.
[0038] Competent Escherichia coli HB101 and BL21(DE)3 were purchased from Beijing Tiangen Company, and other chemical reagents were domestic analytical grade.
[0039] 1.2 Experimental steps
[0040] 1.2.1 ABT clone construction
[0041] In order to obtain the long-acting fusion peptide ABT (SEQ ...
Embodiment 2
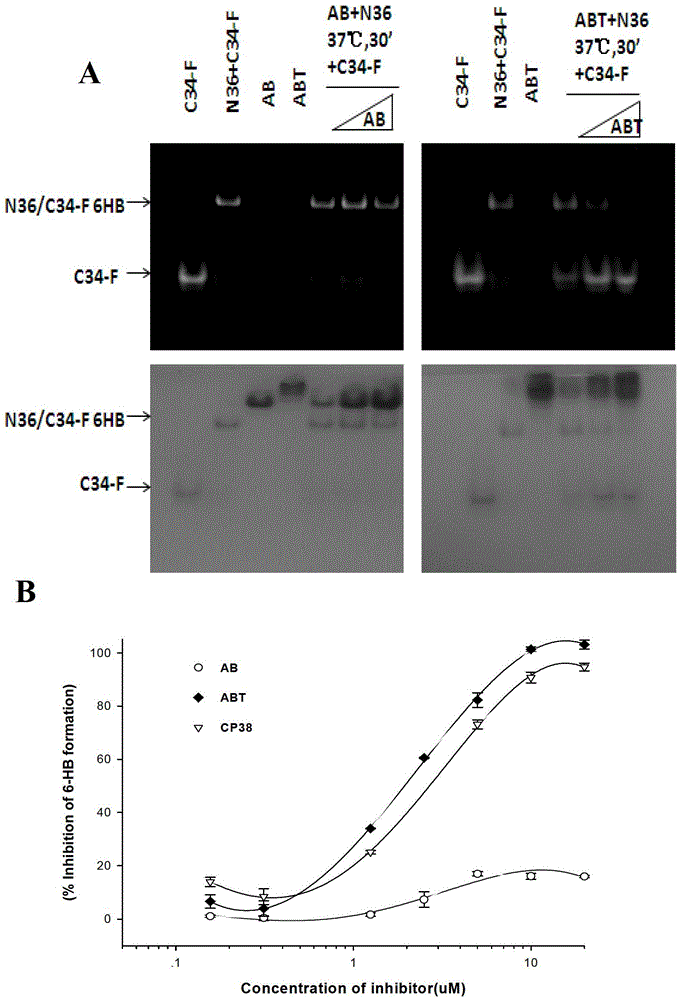
[0059] Embodiment 2: FN‐PAGE detects the influence of AB and ABT on the formation of six helices
[0060] 2.1 Materials
[0061] Non-denaturing polyacrylamide gel (PAGE) gel electrophoresis kit: purchased from Beijing Tianenze Company.
[0062] N36, C34, and FAM-C34 polypeptides were synthesized by Biosystems 433A protein synthesizer.
[0063] 2.2 Experimental process
[0064] (1) Prepare 18% separating gel and 5% stacking gel.
[0065] (2) Prepare peptides such as N36, F‐C34, ABT, AB, N36+ABT, and N36+AB. The final concentration of each peptide is 40 uM, place at 37 degrees for 30 minutes, and avoid light at room temperature. Under the condition of voltage 125V, electrophoresis 2 hours.
[0066] (3) Fluorchem8800 (ultraviolet) detection.
[0067] (4) Coomassie brilliant blue stained PAGE gel.
[0068] This experiment ( figure 2 ) shows that AB does not compete with C34 or N36 to affect the formation of the six helix. Only ABT competes with C34 or N36 to affect the fo...
Embodiment 3
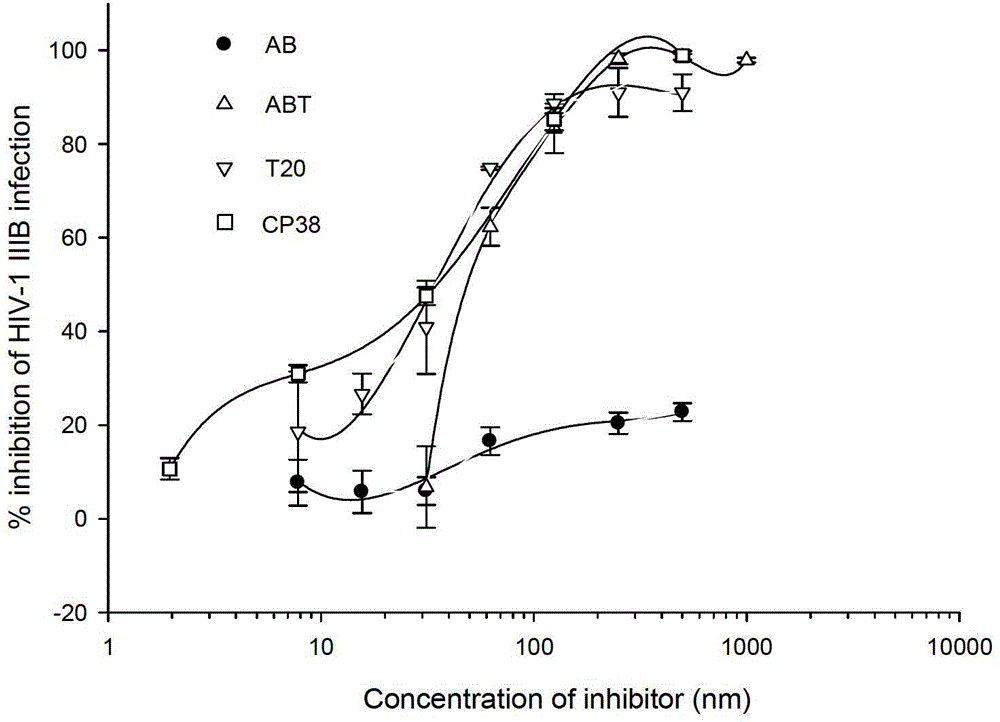
[0069] Embodiment 3: HIV‐1 laboratory-adapted strain and the virus inhibition test of primary virus strain
[0070] 3.1 Experimental materials
[0071] Cells: MT‐2 cells, M7 cells Medium: 1640, 1640+10% FBS.
[0072] Culture plate: 96-well flat culture plate (corning), 96-well round bottom culture plate (corning).
[0073] Cell lysate: 5% TritonX‐100.
[0074] Viruses: laboratory-adapted strains HIV‐1 IIIB, HIV‐1 Bal virus and various HIV‐1 primary virus strains.
[0075] 3.2 Experimental process
[0076] (1) Doubly dilute ABT, AB, CP38 and T20 polypeptide proteins in a 96-well plate, and set positive control wells (no polypeptide protein wells) and negative control wells (cell control wells and virus control wells).
[0077](2) Thoroughly mix the virus strains thawed at -80 degrees Celsius, and add 100 times the TCID50 value (that is, add 50% of the tissue infection dose to the well).
[0078] (3) Adjust the concentration of MT‐2 or M7 cells to 1x105 cells / ml, and add 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com