Novel crystal of tenofovir prodrug
A technology of crystals and prodrugs, applied in the field of medicinal chemistry, can solve the problems of undisclosed compound crystallography, difficult to obtain single crystals, complex crystal distribution, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
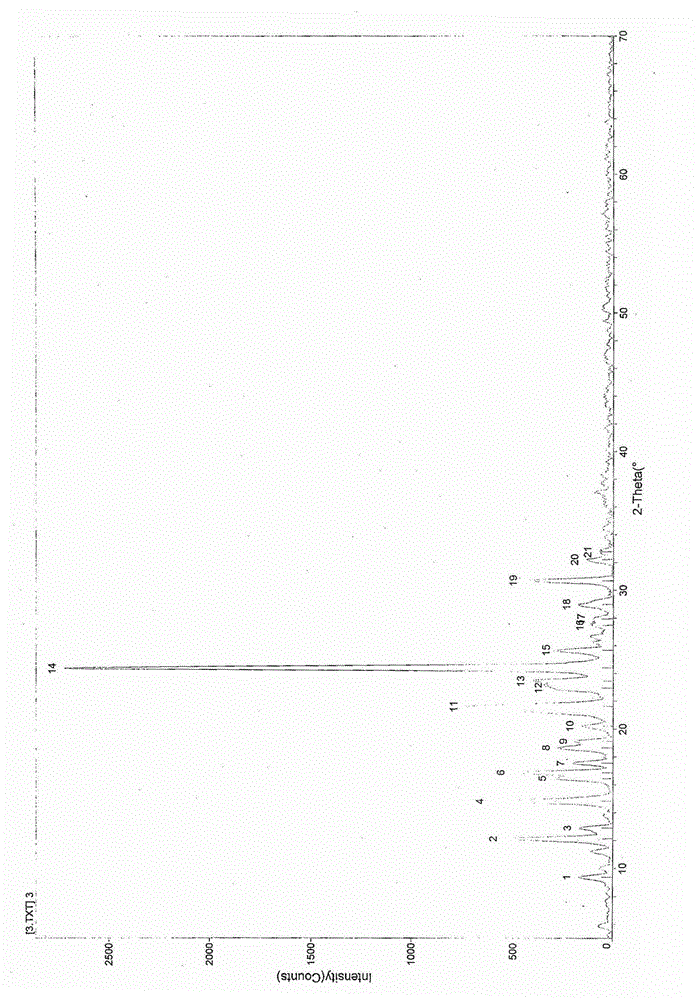
[0029] (2R,4S)-2-((((R)-(6-amino-9-yl)propane-yl)oxy)methyl)-(3-chlorophenyl)-2oxo-1,3,2 – Preparation and identification of Form I crystal form of dioxaphosphorine succinate (HTS)
[0030] Compound Synthesis:
[0031] The steps of compound synthesis basically refer to the preparation method of Chinese Patent No. 200510098771.X to prepare (2R,4S)-2-((((R)-(6-amino-9-yl)propane-yl)oxy)methyl) -(3-Chlorophenyl)-2-oxo-1,3,2-dioxaphosphorinate succinate (HTS). Specific steps are as follows:
[0032]
[0033] Regent
[0034] Tenofovir (1.32g, 4.60mmol) and N,N-dimethylformamide (0.5g, 4.95mmol) were added to 35ml of dichloromethane, and oxalyl chloride (1.4ml) was slowly added dropwise. The solvent was concentrated under reduced pressure to obtain a crude product, which was dissolved in 25ml of dichloromethane, and the temperature was controlled at 0°C, and pyridine (0.75mL, 9.16mmol) was slowly added. Then the temperature was lowered to -78°C, and another dichloro...
Embodiment 2
[0042] Stability Comparison of Crystals of the Invention and Compounds of the Prior Art
[0043] This example describes the comparative experiment on the stability of the crystal of the present invention (type I crystal prepared in Example 1 is numbered as sample 1) and the compound prepared according to the prior art (Chinese patent application 200510098771.X, numbered as sample 2).
[0044] The high-temperature stability test was carried out at 65°C, and the results are shown in the following table (Table 2), which indicates that the crystal of the present invention is more stable at high temperature than the prior art.
[0045] Table 265°C for high temperature stability test
[0046]
[0047] The stability test was carried out at 40°C for 6 months, and the results are shown in the following table (Table 3), which indicates that the crystals of the present invention are more stable under long-term storage than those of the prior art.
[0048] Table 340 ℃ under the stabil...
Embodiment 3
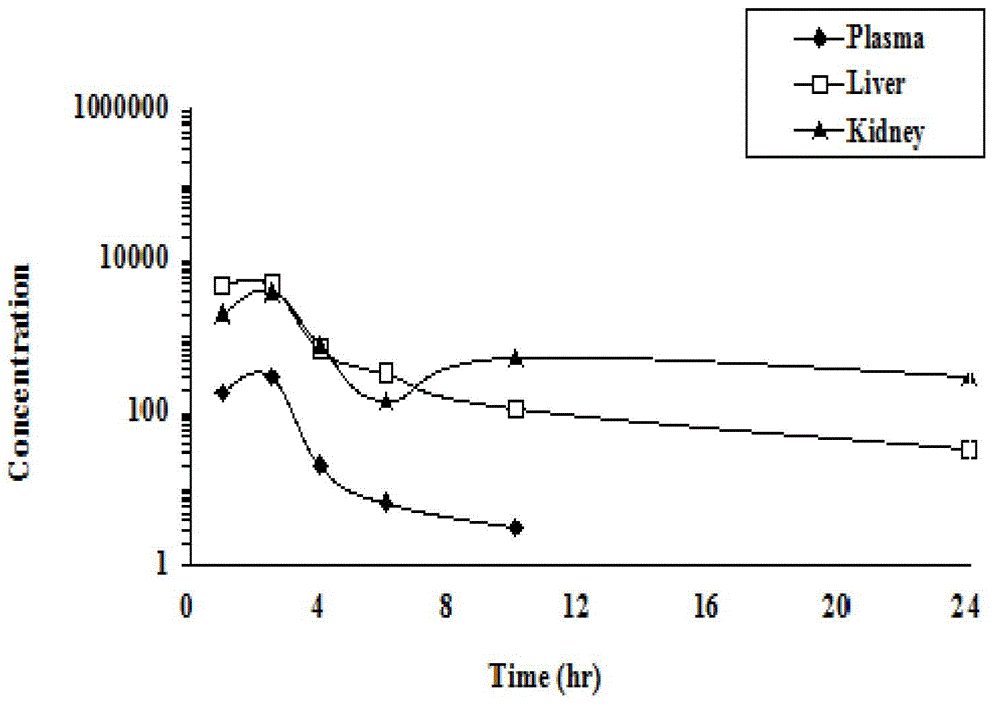
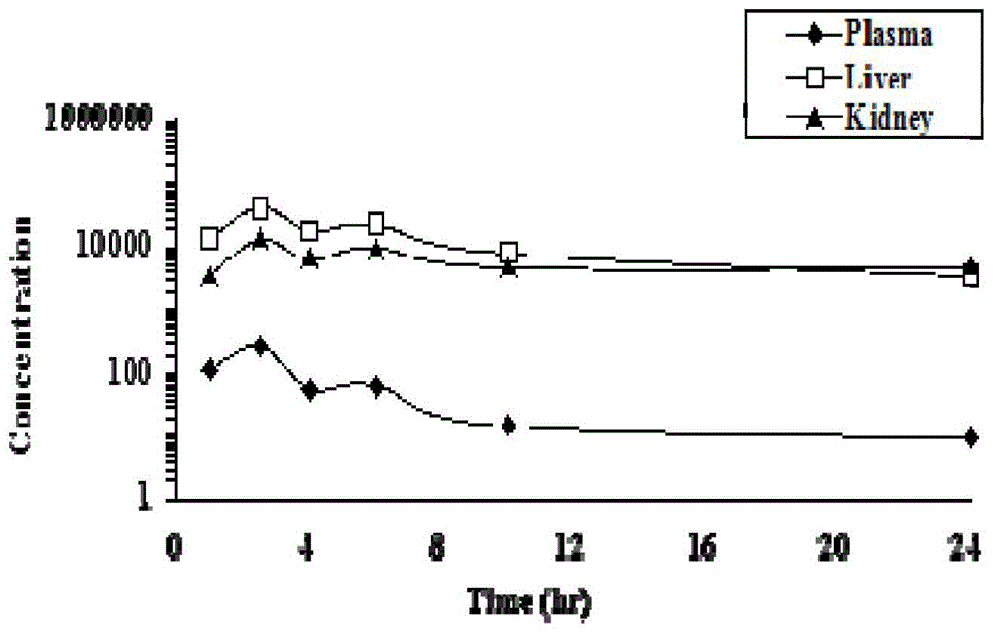
[0051] Comparison of anti-HBV effect of the crystal of the present invention and compounds of the prior art
[0052] This example describes the in vivo metabolism test of the crystal of the present invention (type I crystal prepared in Example 1). Specifically, male Wistar / SD rats (180-210 g) were fasted overnight before administration. Compounds were administered intragastrically in the form of physiological saline (0.9%) solution (5 mg / ml or 10 mg / ml). Administration with 58.06mg / kg HTS (30mg / kg tenofovir equivalent) (n=3 / group), and 66.38mg / kg tenofovir disoproxil fumarate (30mg / kg tenofovir equivalent) as comparison. Animals were anesthetized with halothane at 1.0, 2.5, 4, 6, 10 and 24 hours after dosing. Take blood from the heart, and take liver and kidney tissue for preservation. At the same time, comparing the drug concentration of the control group taking tenofovir disoproxil fumarate during the same period, it was found that (2R,4S)-2-((((R)-(6-amino-9-yl)propane-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com