Preparation method of curcumin derivatives, and antitumor drug
A technology of curcumin derivatives and curcumin, which is applied in the direction of antineoplastic drugs, drug combinations, medical preparations containing active ingredients, etc., can solve the problems of curcumin's poor water solubility, low bioavailability, and fast metabolism in the body, and achieve Good water solubility, high bioavailability, and slow metabolism in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
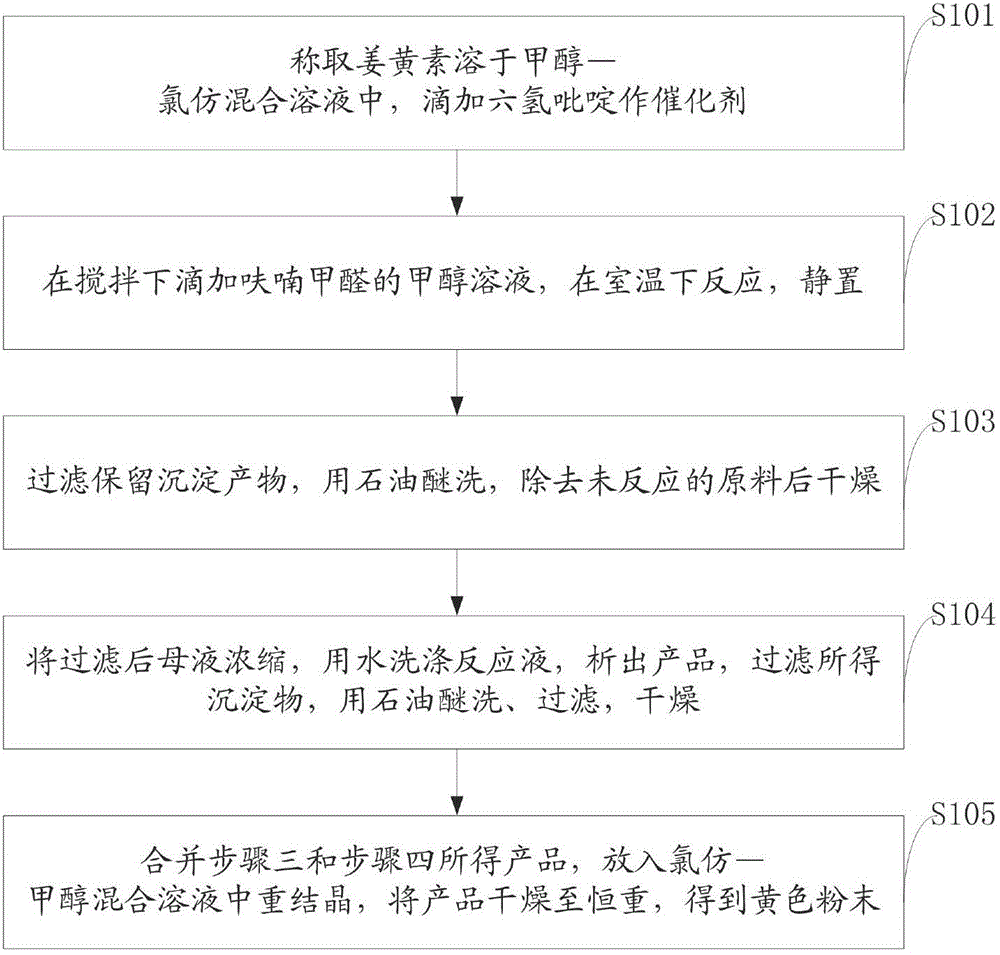
[0054] Example 1 Synthesis of 4-(2-furyl methylene) curcumin.
[0055] Weigh 1.0g (2.72mmol) of curcumin and dissolve it in a mixed solution of methanol-chloroform (methanol 29ml, chloroform 13ml), and add dropwise 0.2ml of hexahydropyridine as a catalyst, and add dropwise a methanol solution of furfuraldehyde (wherein Furfural 0.23ml, about 0.26g, furan formaldehyde redistilled before use), reacted at room temperature for 48h, let the reactant stand still, filtered out the precipitated product (keep the mother liquor), and washed 4 times with 20ml petroleum ether to remove unreacted Raw materials, after drying the product, 0.25g of yellow powder was obtained.
[0056] Concentrate the above mother liquor to about 10ml, wash the reaction solution with water to precipitate the product from the reaction solution, filter the obtained precipitate, wash with 20ml of petroleum ether for 4 times, filter and dry to obtain 0.68g of the product. The products obtained above were combined...
Embodiment 2
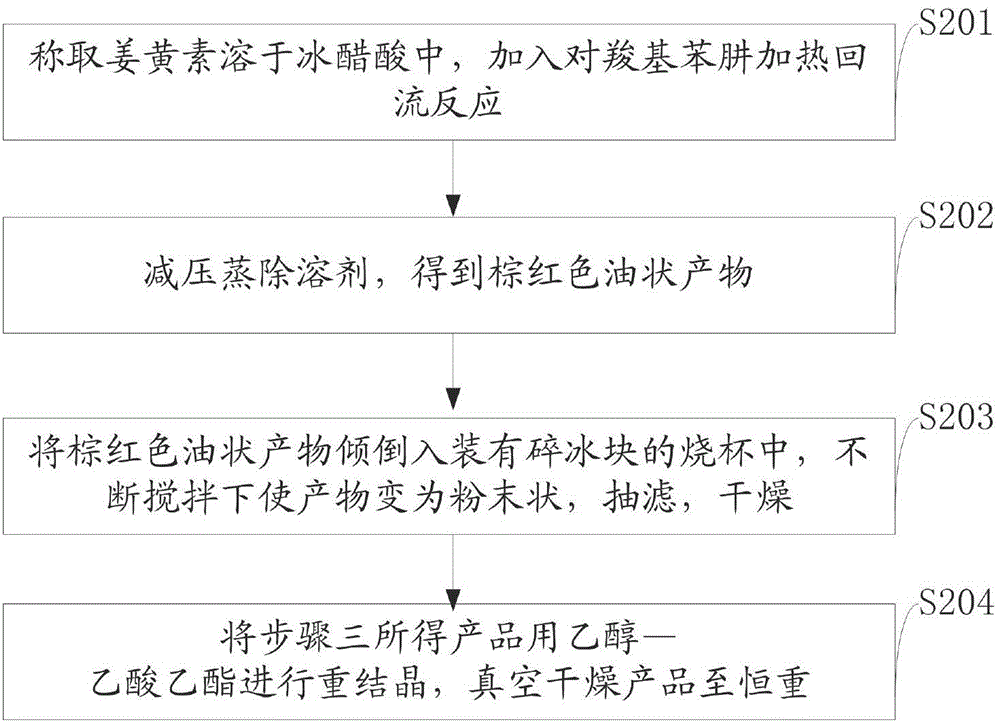
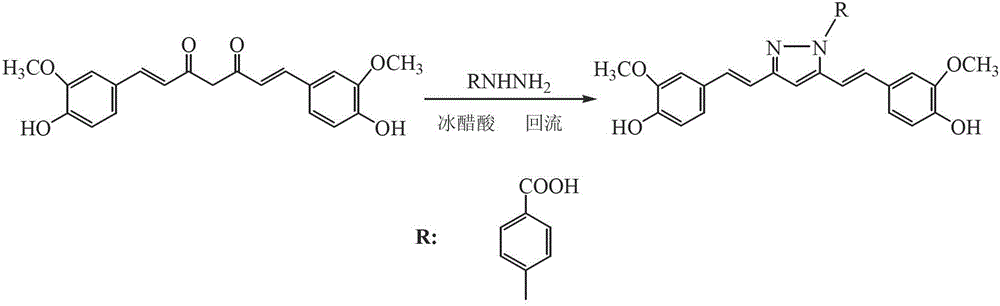
[0059] The synthesis of embodiment two p-carboxyphenylhydrazine curcumin
[0060] Weigh 1.0g (2.72mmol) of curcumin and dissolve it in 35ml of glacial acetic acid, add 0.45g (3.48mmol) of p-carboxyphenylhydrazine and heat to reflux at 120°C for 12 hours. After the reaction was finished, the solvent was evaporated under reduced pressure to obtain a brownish-red oily product, which was poured into a 150ml beaker equipped with crushed ice cubes, and the product was turned into powder under constant stirring, then suction filtered and dried. The product was recrystallized with ethanol:ethyl acetate (5:2), dried in vacuum to constant weight, and weighed 0.85g.
[0061] The product is an orange powder with a yield of 64.4% and a melting point of 157-159°C.
[0062] UV-Vis: λmax (EtOH) 332nm, 429nm. IR(KBr):3381cm -1 (ν OH ),3015cm -1 (ν =CH2 ),2937cm -1 (ν CH2 ),1699cm -1 (ν C=O ),1604cm -1 (ν C=N ),1031cm -1 、1369cm -1 (ν C-H ),792cm -1 、866cm -1 (Meta-substituted ...
Embodiment 3
[0063] The detection of embodiment three compound antitumor activity in vitro
[0064] The tumor cell line used is the human lung cancer A549 cell line. The A549 cells in the logarithmic growth phase were planted in a 96-well plate. After growing to the logarithmic growth phase, drug treatment was added. The control group was added with a certain volume of DMSO, and the experimental group was added with different concentrations of drugs. , placed in an incubator for different periods of time, 4 hours before the end of the drug action time, add 20 μl of MTT stock solution to each well of each group, and place it in the incubator in the dark to continue culturing, discard the supernatant of each well after 4 hours, Add 100 μl DMSO respectively, shake on a shaker at 37°C for 15 minutes, then measure the OD value of each well at 490 nm with a microplate reader, and calculate the cell survival rate: survival rate = (OD experimental group / OD control group) × 100%, activity inhibition...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com