Synthesis method for key intermediate, analogue or salt of ticlopidine
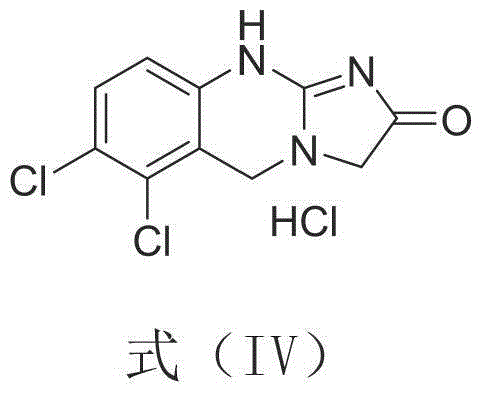
A synthetic method, the technology of anagrelide, applied in the key intermediates of anagrelide, the synthesis of analogues and their salts, analogues and salts and their synthesis, and the field of N-glycine ester, can solve the problem that the yield is only 75%, Complicated operation and other problems, to achieve the effect of high total yield, overcoming the long route, and mild and easy-to-control conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
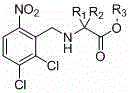
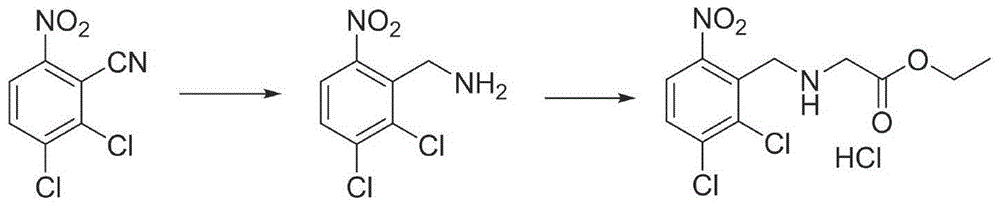
[0046] The preparation of embodiment 1N-(6-nitro-2,3-dichlorobenzyl) glycine
[0047] After mixing 35kg of methanol and 39kg of 2mol / l sodium hydroxide aqueous solution, add 9.2kg of glycine, stir for 20min, dissolve 15.4kg of 2,3-dichloro-6-nitrobenzaldehyde in 35kg of methanol, drop the solution Add it to the above reaction system, control the temperature at 20-25°C, stir for 40min after the dropwise addition, cool down to 0-5°C, and add 120kgNaBH in batches 4 , heated up to 20-25°C and stirred until the reaction was complete, evaporated the methanol under reduced pressure, adjusted the pH to 5 with 2mol / l hydrochloric acid, spin-dried and recrystallized with 30kg of water to obtain 20.78kg of off-white solid with a yield of 97.2%.
Embodiment 2
[0048] The preparation of embodiment 2N-(6-nitro-2,3-dichlorobenzyl) glycine methyl ester
[0049] In a 100L reactor, add 10.0kg N-(6-nitro-2,3-dichlorobenzyl)glycine to 50kg methanol, add 5kg concentrated sulfuric acid, heat and reflux until the reaction is complete, recover methanol under reduced pressure, add 10kg saturated NaHCO 3 The aqueous solution was extracted with ethyl acetate, the organic layer was dried, and the solvent was recovered to obtain 99.8 kg of N-(6-nitro-2,3-dichlorobenzyl) glycine methyl ester, with a yield of 95%.
Embodiment 3
[0050] The preparation of embodiment 3N-(6-nitro-2,3-dichlorobenzyl) glycine methyl ester hydrochloride
[0051] In a 100L reaction kettle, add 10kg of N-(6-nitro-2,3-dichlorobenzyl)glycine to 50kg of methanol, cool down to 0-5°C, add 4.7kg of thionyl chloride dropwise, and the dropwise addition is completed , heated to reflux until the reaction was complete, methanol was reclaimed under reduced pressure, the residue was added to 20 kg of ethyl acetate for beating, and filtered to obtain 11.46 kg of N-(6-nitro-2,3-dichlorobenzyl) glycine hydrochloride. The rate is 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com