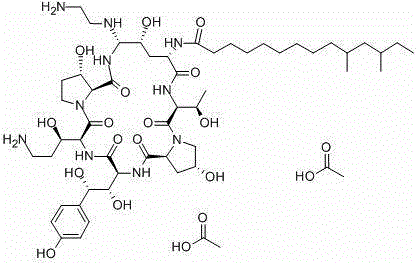
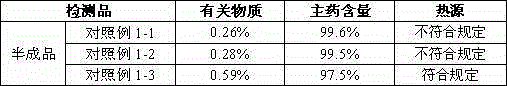
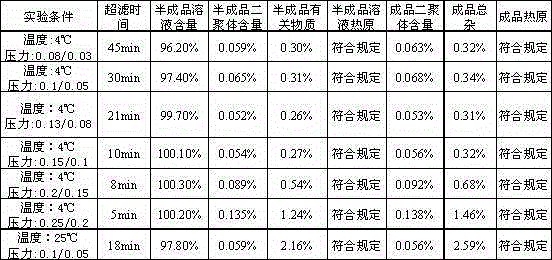
A kind of caspofungin acetate pharmaceutical composition for injection and preparation method thereof
A technology of caspofungin acetate and its composition, which is applied in the field of medicine, can solve the problems of insignificant adsorption of activated carbon, instability of caspofungin acetate, and no pharmacological effect, etc., to avoid poor adsorption effect and polymer impurity content Low, quality-controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] prescription:
[0073] Caspofungin Acetate 5.55g
[0074] Sucrose 3.75g
[0075] Mannitol 2.5g
[0076] Disodium hydrogen phosphate 0.136g
[0077] Sodium dihydrogen phosphate 44mg
[0078] Add water for injection to 125ml
[0079] Take 80% of the prepared water for injection (125ml×80%), add the prescribed amount of sodium dihydrogen phosphate, disodium hydrogen phosphate, sucrose and mannitol, stir and dissolve; cool to a certain temperature, add the prescribed amount of raw material carboacetate Fenjing, stir until completely dissolved, pH 5.6; under certain temperature conditions, ultrafilter the solution through an ultrafiltration system (1.5kd ultrafiltration membrane, control inlet and outlet pressure) into the batching tank, and add water for injection to the full amount; Semi-finished product inspection, fine filtration through 0.22μm microporous membrane, filling, and half-plugging; put the sample in a freeze dryer, cool down to -40~-45°C for 2 hours, and...
Embodiment 2
[0085] prescription:
[0086] Caspofungin Acetate 5.55g
[0087] Sucrose 3.75g
[0088] Mannitol 2.5g
[0089] Disodium hydrogen phosphate 0.136g
[0090] Sodium dihydrogen phosphate 44mg
[0091] Add water for injection to 125ml
[0092] Take 80% of the prepared water for injection (125ml × 80%), add the prescribed amount of sodium dihydrogen phosphate, disodium hydrogen phosphate, sucrose and mannitol, stir and dissolve; cool to about 10°C, add the prescribed amount of raw material carboxy acetate Pofungin, stir until it is completely dissolved, and the pH is 5.6; at a temperature of about 10°C, pass the solution through an ultrafiltration system (1.5kd ultrafiltration membrane, the inlet pressure is controlled at 0.15Mpa, and the outlet pressure is controlled at 0.1Mpa). Fill the batching tank with water for injection to the full amount; test the semi-finished product, filter it through a 0.22μm microporous membrane after passing the test, fill it, and press half the s...
Embodiment 3
[0094] prescription:
[0095] Caspofungin Acetate 5.55g
[0096] Sucrose 3.75g
[0097] Mannitol 2.5g
[0098] Disodium hydrogen phosphate 0.136g
[0099] Sodium dihydrogen phosphate 44mg
[0100] Add water for injection to 125ml
[0101] Take 80% of the prepared water for injection (125ml×80%), add the prescribed amount of sodium dihydrogen phosphate, disodium hydrogen phosphate, sucrose and mannitol, stir to dissolve; cool to about 8°C, add the prescribed amount of raw materials, and stir Until it is completely dissolved, the pH value is 5.8; at a temperature of about 8°C, pass the solution through an ultrafiltration system (2.0kd ultrafiltration membrane, the inlet pressure is controlled at 0.15Mpa, and the outlet pressure is controlled at 0.1Mpa) into the batching tank. Replenish the water for injection to the full amount; test the semi-finished product, filter it through a 0.22 μm microporous membrane after passing the test, fill it, and press half the stopper; put t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com