Phenol derivatives containing (Sp)-2-chiral phosphinate substituent groups and preparation method thereof
A technology of phosphinate and substituents, which is applied in the field of phenolic derivatives containing 2-chiral phosphinate substituents and the preparation thereof, can solve the problem of difficult recycling of chiral resolution reagents, resolution reagents Low activity and selectivity, harsh reaction conditions, etc., to achieve good industrial application prospects, cheap catalysts, and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
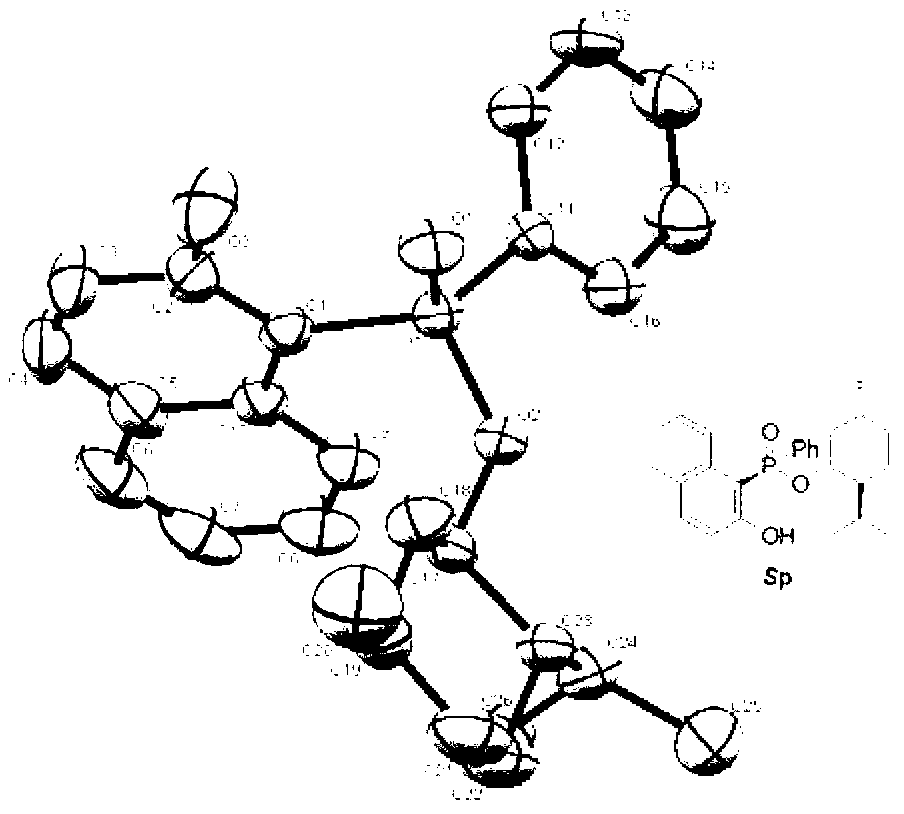
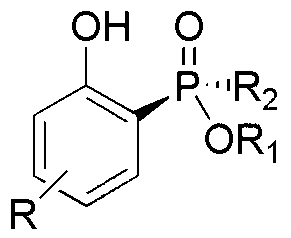
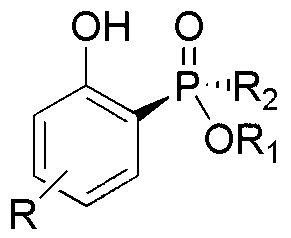
[0028] Take 0.280 g (1.0 mmol) Rp-phenylphosphinic acid-(-)-menthol ester, 220 mg (1.0 mmol) 2-iodo-phenol, 489 mg (1.5 mmol) cesium carbonate, 2.0 mL N,N- Add dimethylformamide and 3.0 mL toluene to the Schlenk tube under nitrogen atmosphere, add 115.6 mg (0.1 mmol) palladium tetrakistriphenylphosphine, o The reaction was stirred at C for 12 h to obtain the target product Sp-(-)-menthol-2-hydroxyphenyl-phenylphosphinate with a yield of 73%.
Embodiment 2
[0030] Take 0.280 g (1.0 mmol) Rp-phenylphosphinic acid-(-)-menthol ester, 226.6 mg (1.03 mmol) 2-iodo-phenol, 212 mg (1.0 mmol) potassium phosphate and 5.0 mL toluene in a nitrogen atmosphere Add 5.94 mg (0.06 mmol) cuprous chloride to the Schlenk tube at 100 o The reaction was stirred at C for 13 h to obtain the target product Sp-(-)-menthol-2-hydroxyphenyl-phenylphosphinate with a yield of 76%.
Embodiment 3
[0032] Take 0.280 g (1.0 mmol) Rp-phenylphosphinic acid-(-)-menthol ester, 231 mg (1.05 mmol) 2-iodo-phenol, 179.4 mg (1.3 mmol) potassium carbonate and 3.0 mL 1,4- Dioxane was added to the Schlenk tube under nitrogen atmosphere, and 11.4 mg (0.08 mmol) of cuprous bromide was added, at 80 o The reaction was stirred at C for 16 h to obtain the target product Sp-(-)-menthol-2-hydroxyphenyl-phenylphosphinate with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com