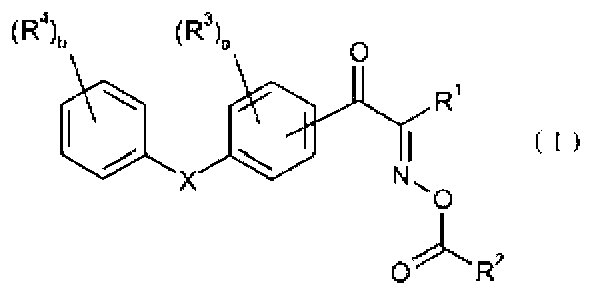
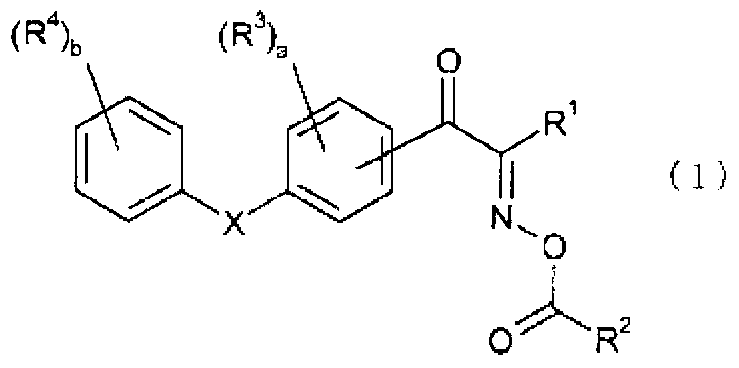
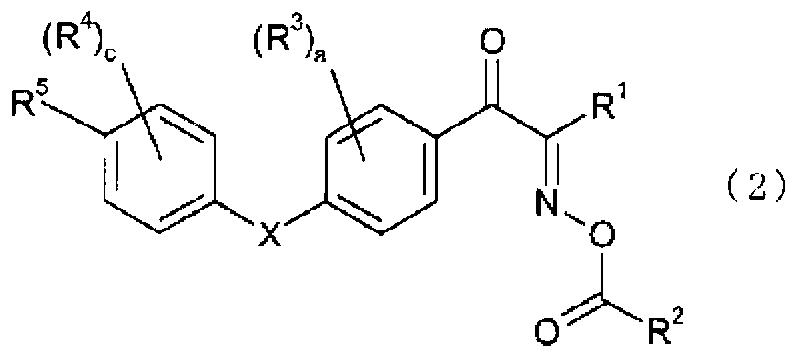
Oxime ester compound and photoinitiator containing said compound
A technology for photopolymerization initiators and compounds, which is applied in the fields of compounds, optics, filters, etc. of Group 5/15 elements of the periodic table, can solve the problems of photomask and heating furnace pollution, residues, etc., and improve heat resistance. Sex, the effect of less sublimation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] [Example 1] Production of compound No.1
[0150] Methacrylate
[0151] 19.8g (65mmol) of 1-(4-(4-(2-hydroxyethoxy)phenylthio)phenyl)propan-1-one (hereinafter also referred to as ketone body a) and 56.2g of methacrylic acid (653 mmol), 5.0 g (26 mmol) of p-toluenesulfonic acid monohydrate, 1.2 g (5.6 mmol) of BHT and 121 g of dichloroethane were stirred and refluxed for 10.5 hours. After cooling, ethyl acetate and water were added for oil-water separation. The organic layer was washed sequentially with water, aqueous sodium bicarbonate solution, and water. After the organic layer was dried over anhydrous magnesium sulfate, solvent removal was performed to obtain 26.3 g of the following methacrylate body a.
[0152]
[0153] Oximation
[0154] Cool the solution containing 26.0g (70mmol) of methacrylate body a obtained in step 1, 7.3g (70mmol) of concentrated hydrochloric acid and 70g of dimethylformamide to 5°C, and add 10.8g of isobutyl nitrite ( 105mmol), stir...
Embodiment 2~4
[0158] [Examples 2-4] Production of Compound No.2, Compound No.3, and Compound No.4
[0159] Use the corresponding ketone body instead of ketone body a (and for compound No.3, use acrylic acid instead of methacrylic acid), except that, according to the method described in Example 1, compound No.2, compound No.3 and compound No.4. The analysis results are shown in [Table 1] to [Table 3]. lambda
[0160] Table 1
[0161]
[0162] *1: CHCl is used as the solvent 3
[0163] Table 2
[0164]
[0165] table 3
[0166]
[0167]
Embodiment 5
[0168] [Example 5] Manufacture of alkali-developable photosensitive resin composition No.1
[0169] Preparation of alkali-developable resin [alkali-developable (B) component]
[0170] Add 184g of bisphenol fluorene type epoxy resin (epoxy equivalent of 231, the epoxy compound represented by the above general formula (3)), 58g of acrylic acid, 0.26 of 2,6-di-tert-butyl-p-cresol into the reaction vessel g, 0.11 g of tetra-n-butylammonium bromide, and 23 g of propylene glycol-1-monomethyl ether-2-acetate were stirred at 120° C. for 16 hours. The reaction solution was cooled to room temperature, 35 g of propylene glycol-1-monomethyl ether-2-acetate, 59 g of biphenyltetracarboxylic anhydride, and 0.24 g of tetra-n-butylammonium bromide were added, and stirred at 120° C. for 4 hours. Furthermore, 20 g of tetrahydrophthalic anhydride was added, and stirred at 120° C. for 4 hours, at 100° C. for 3 hours, at 80° C. for 4 hours, at 60° C. for 6 hours, and at 40° C. for 11 hours. hour...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com