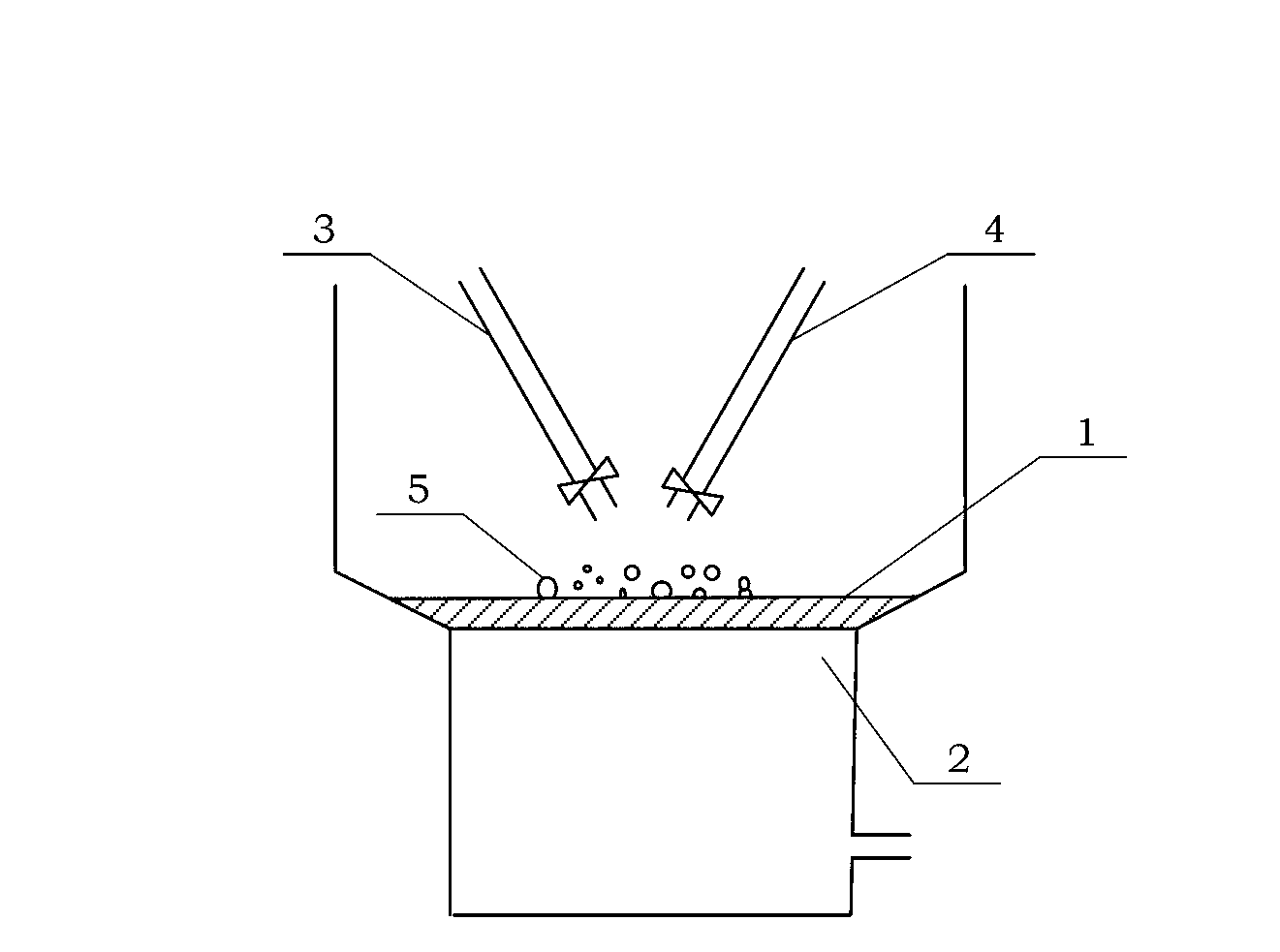
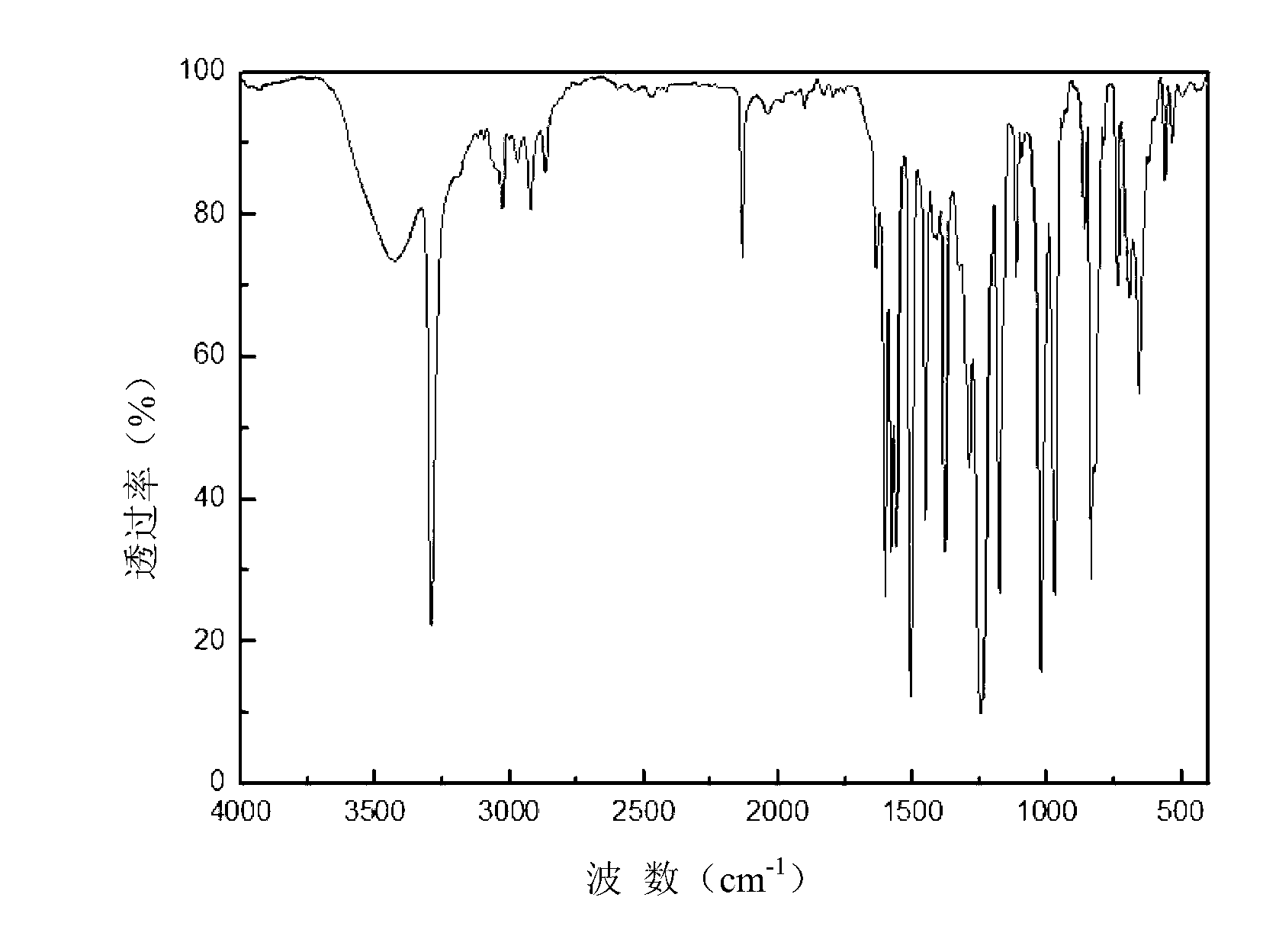
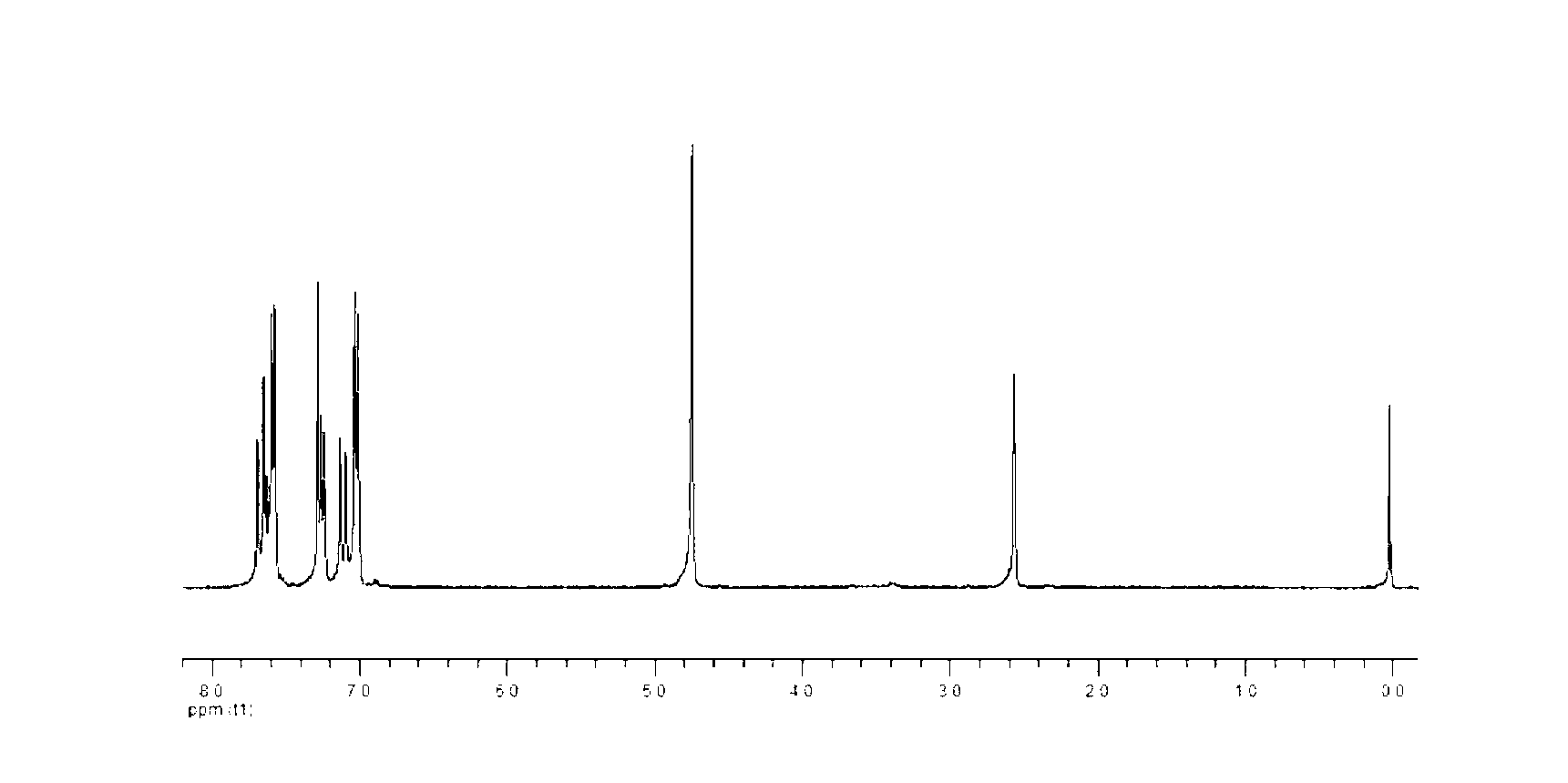
Propargyl ether resin with main chain containing pyridine ring aromatic bisphenol-type terminal and preparation method thereof
A pyridine ring aromatic, terminal propargyl ether technology, applied in the field of high temperature resistant resin, to achieve the effects of good fluidity, high yield and excellent heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] 2,6-bis(4-propargyl-oxy-styryl)pyridine is prepared by phase transfer catalytic reaction method, the specific preparation steps are as follows:
[0069] (A) Prepare an aqueous sodium hydroxide solution with a concentration of 20% by mass;
[0070] (B) Mix 2,6-bis(4-hydroxystyryl)pyridine, aqueous sodium hydroxide solution and tetrabutylammonium bromide to obtain a mixed solution;
[0071] Dosage: Add 1000 mL of sodium hydroxide aqueous solution and 0.1 mol of tetrabutylammonium bromide to 1mol of 2,6-bis(4-hydroxystyryl)pyridine;
[0072] The structural formula of the 2,6-bis(4-hydroxystyryl)pyridine is as shown in formula 3:
[0073] Formula 3
[0074] In formula 3, HO is a hydroxyl group, OH is a hydroxyl group, the hydroxyl group is at the para position of the vinyl HCHC, A is the vinyl HCHC; Y is hydrogen.
[0075] The structure of Formula 3 in Example 1 can be prepared according to the method disclosed in the "NEW STYRYLPWRIDINE-BASED POLYESTERS AND POLYURETHANES" document. ...
Embodiment 2
[0105] The phase transfer catalytic reaction method is used to prepare 2,6-bis(4-propargyl-oxy-benzoyl)pyridine. The specific steps are as follows:
[0106] (A) Prepare an aqueous potassium hydroxide solution with a concentration of 15% by mass;
[0107] (B) Mix 2,6-bis(4-hydroxybenzoyl)pyridine, potassium hydroxide aqueous solution and benzyltrimethylammonium bromide to obtain a mixed solution;
[0108] Dosage: Add 1500 mL of potassium hydroxide aqueous solution and 0.12 mol of benzyltrimethylammonium bromide to 1mol of 2,6-bis(4-hydroxybenzoyl)pyridine;
[0109] The structural formula of the 2,6-bis(4-hydroxybenzoyl)pyridine is as shown in formula 5:
[0110] Formula 5
[0111] In formula 5, HO is a hydroxyl group, OH is a hydroxyl group, and a hydroxyl group is at a carbonyl group. The para position, A is the carbonyl group Y is hydrogen.
[0112] The structure of Formula 5 in Example 2 can be prepared according to the method disclosed in the document "Synthesis and Performance Rese...
Embodiment 3
[0142] The phase transfer catalytic reaction method is used to prepare 4-chloro-2,6-bis(4-propargyl-oxy-styryl)pyridine. The specific steps are as follows:
[0143] (A) Prepare an aqueous solution of sodium hydroxide with a concentration of 30% by mass;
[0144] (B) Combine 4-chloro-2,6-bis(4-hydroxystyryl)pyridine, aqueous sodium hydroxide and tetraethylammonium bromide to obtain a mixed solution;
[0145] Dosage: 500mL of sodium hydroxide aqueous solution and 0.008mol of tetraethylammonium bromide are added to 1mol of 4-chloro-2,6-bis(4-hydroxystyryl)pyridine;
[0146] The structural formula of the 4-chloro-2,6-bis(4-hydroxystyryl)pyridine is as shown in formula 7:
[0147] Formula 7
[0148] In formula 7, HO is a hydroxyl group, OH is a hydroxyl group, and the hydroxyl group is at the para position of vinyl-HC=HC-, A is vinyl*HC=HC-, and Y is chlorine.
[0149] The structure of Formula 7 in Example 3 can be prepared according to the method disclosed in the "NEW STYRYLPWRIDINE-BASED PO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com