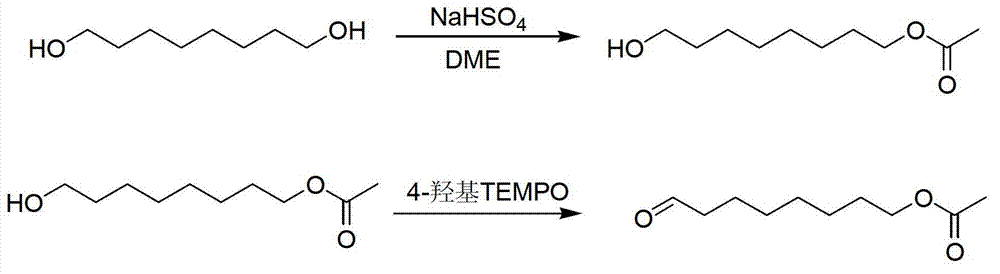
Method for synthesizing 8-acetoxyl octaldehyde
A technology of acetoxy octanal and acetoxy octanol is applied in the synthesis field of 8-acetoxy octanal, an important intermediate of 10-hydroxy-2-decenoic acid, and can solve the influence of residual solvent and the influence of subsequent reactions , difficulties in suction filtration, etc., to achieve the effect of simplified process synthesis route, mild reaction conditions, and economical and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Step 1) In a 2000mL three-necked flask equipped with a mechanical stirrer, a thermometer, and a constant pressure dropping funnel, add 1200mL of DME, 65mL of acetic acid, and 100mL of deionized water in sequence, mix them well, and add 1,8-octanediol to the above system 150 g and 10 g of sodium bicarbonate, the temperature was raised to react for 5 hours.
[0027] Step 2) Cool down to room temperature after the reaction, separate the water layer, wash the organic layer with water until neutral, evaporate the solvent to obtain crude 8-acetoxyoctanol.
[0028] Step 3) Add 100g of 8-acetoxyoctanol, 2g of KBr, and 300g of sodium bicarbonate into 800mL of dichloromethane, keep the temperature at 20°C, and gradually add 100g of 4-hydroxy TEMPO. React for 1 hour. After the reaction, wash the 8-acetoxyoctylal with water, add anhydrous magnesium sulfate to the organic layer, dry it, and filter it with suction. The dichloromethane is evaporated from the solution under normal pres...
Embodiment 2
[0032] Step 1) In a 2000mL three-necked flask equipped with a mechanical stirrer, a thermometer and a constant pressure dropping funnel, add 800mL of DME, 50mL of acetic acid, and 80mL of deionized water in sequence, mix them well, and add 1,8-octanediol to the above system 150 g and 10 g of sodium bicarbonate, the temperature was raised to react for 5 hours.
[0033] Step 2) Cool down to room temperature after the reaction, separate the water layer, wash the organic layer with water until neutral, evaporate the solvent to obtain crude 8-acetoxyoctanol.
[0034] Step 3) Add 100g of 8-acetoxyoctanol, 1g of KBr, and 300g of sodium bicarbonate into 500mL of dichloromethane, keep the temperature at 10°C, and gradually add 90g of 4-hydroxy TEMPO. React for 2 hours. After the reaction is over, wash 8-acetoxyoctanal with water, add anhydrous magnesium sulfate to the organic layer, dry it, and filter it with suction. The solution is evaporated to dichloromethane under normal pressure ...
Embodiment 3
[0036] Step 1) In a 2000mL three-necked flask equipped with a mechanical stirrer, a thermometer, and a constant pressure dropping funnel, add 1200mL of DME, 80mL of acetic acid, and 120mL of deionized water, mix them well, and add 1,8-octanediol to the above system 180g and 10g of sodium bicarbonate, the temperature was raised to react for 5 hours.
[0037] Step 2) Cool down to room temperature after the reaction, separate the water layer, wash the organic layer with water until neutral, evaporate the solvent to obtain crude 8-acetoxyoctanol.
[0038] Step 3) Add 100g of 8-acetoxyoctanol, 10g of KBr, and 500g of sodium bicarbonate into 800mL of dichloromethane, keep the temperature at 30°C, and gradually add 100g of 4-hydroxy TEMPO. The reaction was carried out for 0.5 hours. After the reaction, the 8-acetoxyoctylal was washed with water, the organic layer was dried by adding anhydrous magnesium sulfate, and then suction-filtered. The dichloromethane was evaporated from the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com