Genistein positive ion nano lipid carrier and preparation method thereof
A technology of nano-lipid carrier and genistein, which can be applied in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The problems of drug dosage and low solubility of genistein have achieved good industrialization prospects, good market prospects, and the effect of increasing drug loading.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
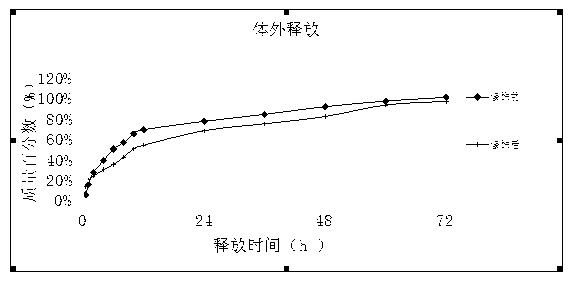
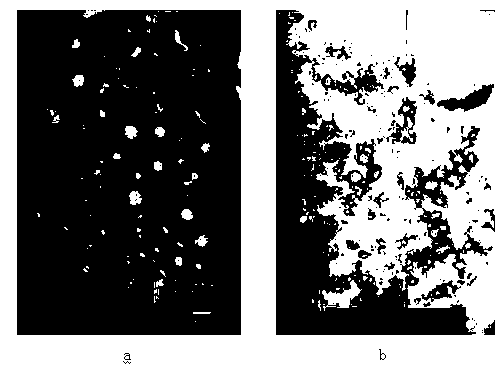
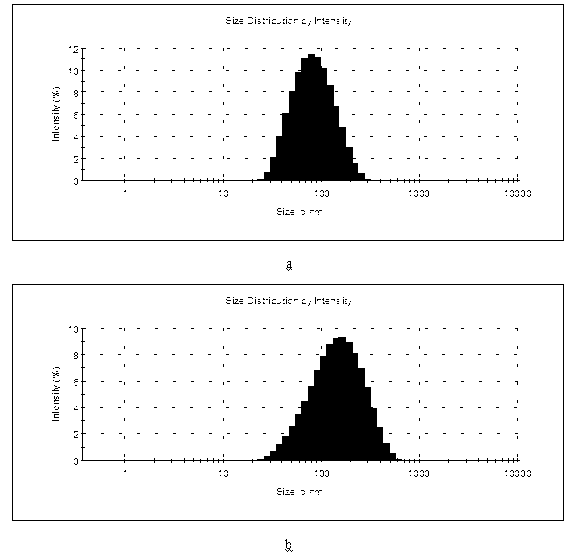
Image
Examples
Embodiment 1
[0039] Example 1: Accurately weigh 10 mg of genistein, 150 mg of glyceryl behenate, 100 mg of glyceryl caprylate, and 20 mg of macrogolglyceride neodecanoate, mix evenly, and heat to 85°C to form an oil phase; Weigh 100mg of soybean lecithin, 300mg of polyoxyethylene castor oil and ultrapure water (the final concentration of phospholipids is 0.75%, w / v) and mix evenly at 85°C to form a water phase; stir at 90°C at 400-600r / min Slowly add the water phase to the oil phase and mix under the high speed. After the dropwise addition, continue to stir and emulsify for 10 minutes. While maintaining the temperature of the colostrum system, use an ultrasonic breaker with a power of 200w to perform ultrasonic crushing for 2 minutes; transfer the sonicated colostrum to an ice bath for low-temperature solidification. Slowly add the prepared nano-lipid carrier dropwise at a stirring speed of 400-600r / min to a concentration of 0.1mg / mL of methacrylic acid and methacrylate copolymer (ethyl ac...
Embodiment 2
[0040] Example 2: Accurately weigh 10 mg of genistein, 120 mg of glyceryl monostearate, 80 mg of soybean oil, and 20 mg of macrogolglyceride neodecanoate, mix evenly, and heat to 85°C to form an oil phase; Soybean lecithin 75mg, polyethylene glycol lauryl hydroxystearate 250mg and ultrapure water (the final concentration of phospholipids is 0.5%, w / v) are mixed uniformly at 85°C to form a water phase; At a stirring speed of 1 / min, slowly add the water phase to the oil phase and mix, after the dropwise addition, continue to stir and emulsify for 10 min. While maintaining the temperature of the colostrum system, use an ultrasonic breaker with a power of 400w to perform ultrasonic crushing for 2 minutes; transfer the sonicated colostrum to an ice bath for low-temperature solidification. Slowly add the prepared nano-lipid carrier dropwise at a stirring speed of 400-600r / min to ethyl enoate, methyl methacrylate and trimethylethyl methacrylate at a concentration of 0.5 mg / mL (1:2:0...
Embodiment 3
[0041] Example 3: Accurately weigh 5 mg of genistein, 80 mg of glycerol palmitostearate, 50 mg of oleic acid, and 10 mg of macrogol glycerol oleate, mix evenly, and heat to 85° C. to form an oil phase; accurately weigh Soybean lecithin 50mg, Tween-80225mg and ultrapure water (the final concentration of phospholipids is 0.75%, w / v) are uniformly mixed at 85°C to form a water phase; The water phase was slowly added into the oil phase for mixing, and after the dropwise addition was completed, the stirring and emulsification was continued for 10 minutes. While maintaining the temperature of the colostrum system, use an ultrasonic breaker with a power of 200w to perform ultrasonic crushing for 2 minutes; transfer the sonicated colostrum to an ice bath for low-temperature solidification. The prepared nano-lipid carrier was slowly added dropwise at a stirring speed of 400-600r / min to a concentration of 1.0mg / mL ethyl acrylate methyl methacrylate and trimethylaminoethyl methacrylate c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com